NGF signals through TrkA to increase clathrin at the plasma membrane and enhance clathrin-mediated membrane trafficking
- PMID: 11007890
- PMCID: PMC6772792
- DOI: 10.1523/JNEUROSCI.20-19-07325.2000
NGF signals through TrkA to increase clathrin at the plasma membrane and enhance clathrin-mediated membrane trafficking
Abstract
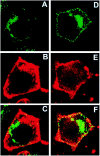
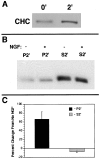
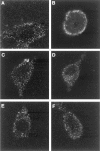
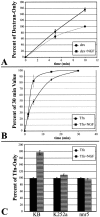
Neurotrophin (NT) signals may be moved from axon terminals to neuron cell bodies via signaling endosomes-organelles in which NTs continue to be bound to their activated receptors. Suggesting that clathrin-coated membranes serve as one source of signaling endosomes, in earlier studies we showed that nerve growth factor (NGF) treatment increased clathrin at the plasma membrane and resulted in colocalization of clathrin with TrkA, the receptor tyrosine kinase for NGF. Strikingly, however, we also noted that most clathrin puncta at the surface of NGF-treated cells did not colocalize with TrkA, raising the possibility that NGF induces a general increase in clathrin-coated membrane formation. To explore this possibility further, we examined the distribution of clathrin in NGF- and BDNF-treated cells. NGF signaling in PC12 cells robustly redistributed the adaptor protein AP2 and the clathrin heavy chain (CHC) to surface membranes. Using confocal and epifluorescence microscopy, as well as biochemical assays, we showed the redistribution of clathrin to be attributable to the activation of TrkA. Significantly, NGF signaled through TrkA to induce an increase in clathrin-mediated membrane trafficking, as revealed in the increased endocytosis of transferrin. In that BDNF treatment increased AP2 and clathrin at the surface membranes of hippocampal neurons, these findings may represent a physiologically significant response to NTs. We conclude that NT signaling increases clathrin-coated membrane formation and clathrin-mediated membrane trafficking and speculate that this effect contributes to their trophic actions via the increased internalization of receptors and other proteins that are present in clathrin-coated membranes.
Figures




References
-
- Beattie EC, Zhou J, Grimes ML, Bunnett NW, Howe CL, Mobley WC. A signaling endosome hypothesis to explain NGF actions: potential implications for neurodegeneration. Cold Spring Harb Symp Quant Biol. 1996;61:389–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
