Mechanisms mediating pituitary adenylate cyclase-activating polypeptide depolarization of rat sympathetic neurons
- PMID: 11007893
- PMCID: PMC6772758
- DOI: 10.1523/JNEUROSCI.20-19-07353.2000
Mechanisms mediating pituitary adenylate cyclase-activating polypeptide depolarization of rat sympathetic neurons
Abstract
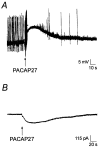
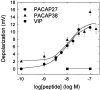
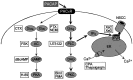
The direct effects of pituitary adenylate cyclase-activating polypeptides (PACAP) on sympathetic neurons were investigated using rat superior cervical ganglion neurons. Electrophysiological and pharmacological analyses were used to evaluate PACAP modulation of sympathetic neuron membrane potentials and to investigate potential ionic and intracellular signaling mechanisms mediating the responses. More than 90% of the sympathetic neurons were depolarized by the PACAP peptides even when stimulated release was blocked, indicating that the PACAP peptides elicited primary responses in the postganglionic neurons. The response profile was consistent for activation of PACAP-selective PAC(1) receptors: nanomolar concentrations of PACAP27 and PACAP38 were required to stimulate depolarization, whereas vasoactive intestinal peptide failed to evoke any response. Furthermore, depolarizations elicited by PACAP27 were reduced by the PAC(1) receptor antagonist PACAP(6-38). Both sodium influx and inhibition of a potassium current contributed to the peptide-induced depolarizations. Activation of neither pertussis toxin- nor cholera toxin-sensitive G-proteins was required for generation of the depolarizations. cAMP and diacylglycerol production and activation of protein kinase A or protein kinase C also were not requisite for the responses. By contrast, phospholipase C (PLC)-dependent inositol 1,4,5-triphosphate (IP(3)) synthesis was crucial to the PACAP-mediated depolarizations. Although calcium release from IP(3)-sensitive stores was not required for the PACAP-induced responses, inhibition of IP(3) receptors reduced the depolarizations. Thus, among the many signal transduction pathways coupled to the PAC(1) receptor, the PACAP-induced depolarization of sympathetic neurons appears to require activation of PLC and subsequent generation of IP(3).
Figures


References
-
- Absood A, Chen D, Hakanson R. Neuropeptides of the vasoactive intestinal peptide/helodermin/pituitary adenylate cyclase activating peptide family elevate plasma cAMP in mice: comparison with a range of other regulatory peptides. Regul Pept. 1992;40:311–322. - PubMed
-
- Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–331. - PubMed
-
- Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol. 1995;16:53–88. - PubMed
-
- Beaudet MM, Braas KM, May V. Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J Neurobiol. 1998;36:325–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
