Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study
- PMID: 11007903
- PMCID: PMC6772793
- DOI: 10.1523/JNEUROSCI.20-19-07438.2000
Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study
Abstract
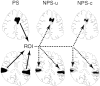
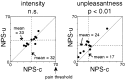
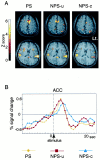
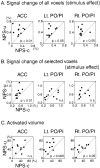
Although behavioral studies suggest that pain distress may alter the perception of somatic stimulation, neural correlates underlying such alteration remain to be clarified. The present study was aimed to test the hypothesis that expectation of pain might amplify brain responses to somatosensory stimulation in the anterior cingulate cortex (ACC) and the region including parietal operculum and posterior insula (PO/PI), both of which may play roles in regulating pain-dependent behavior. We compared brain responses with and subjective evaluation of physically identical nonpainful warm stimuli between two psychologically different contexts: one linked with pain expectation by presenting the nonpainful stimuli randomly intermixed with painful stimuli and the other without. By applying the event-related functional magnetic resonance imaging technique, brain responses to the stimuli were assessed with respect to signal changes and activated volume, setting regions of interest on activated clusters in ACC and bilateral PO/PI defined by painful stimuli. As a result, the uncertain expectation of painful stimulus enhanced transient brain responses to nonpainful stimulus in ACC and PO/PI. The enhanced responses were revealed as a higher intensity of signal change in ACC and larger volume of activated voxels in PO/PI. Behavioral measurements demonstrated that expectation of painful stimulus amplified perceived unpleasantness of innocuous stimulus. From these findings, it is suggested that ACC and PO/PI are involved in modulation of affective aspect of sensory perception by the uncertain expectation of painful stimulus.
Figures


References
-
- Barsky AJ, Borus JF. Functional somatic syndromes. Ann Intern Med. 1999;130:910–921. - PubMed
-
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med. 1999;41:1044–1057. - PubMed
-
- Berthier M, Starkstein S, Leiguarda R. Asymbolia for pain: a sensory-limbic disconnection syndrome. Ann Neurol. 1988;24:41–49. - PubMed
-
- Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802–807. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
