Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus
- PMID: 11007904
- PMCID: PMC6772787
- DOI: 10.1523/JNEUROSCI.20-19-07446.2000
Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus
Abstract
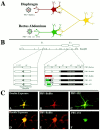
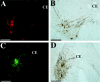
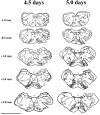
During a number of behaviors, including vomiting and some postural adjustments, activity of both the diaphragm and abdominal muscles increases. Previous transneuronal tracing studies using injection of pseudorabies virus (PRV) into either the diaphragm or rectus abdominis (RA) of the ferret demonstrated that motoneurons innervating these muscles receive inputs from neurons in circumscribed regions of the spinal cord and brainstem, some of which have an overlapping distribution in the magnocellular part of the medullary reticular formation (MRF). This observation raises two possibilities: that two populations of MRF neurons provide independent inputs to inspiratory and expiratory motoneurons or that single MRF neurons have collateralized projections to both groups of motoneurons. The present study sought to distinguish between these prospects. For this purpose, recombinant isogenic strains of PRV were injected into these respiratory muscles in nine ferrets; the strain injected into the diaphragm expressed beta-galactosidase, whereas that injected into RA expressed green fluorescent protein. Immunofluorescence localization of the unique reporters of each virus revealed three populations of infected premotor neurons, two of which expressed only one virus and a third group that contained both viruses. Dual-infected neurons were predominantly located in the magnocellular part of the MRF, but were absent from both the dorsal and ventral respiratory cell groups. These data suggest that coactivation of inspiratory and expiratory muscles during behaviors such as emesis and some postural adjustments can be elicited through collateralized projections from a single group of brainstem neurons located in the MRF.
Figures




References
-
- Bartha A. Experimental reduction of virulence of Aujezky's disease. Magy Allatorv Lapja. 1961;16:42–45.
-
- Bianchi AL, Grèlot L. Converse motor output of inspiratory bulbospinal premotoneurones during vomiting. Neurosci Lett. 1989;104:298–302. - PubMed
-
- Billig I, Foris JM, Card JP, Yates BJ. Transneuronal tracing of neural pathways controlling an abdominal muscle, rectus abdominis, in the ferret. Brain Res. 1999;820:31–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
