Cortical feedback controls the frequency and synchrony of oscillations in the visual thalamus
- PMID: 11007907
- PMCID: PMC6772790
- DOI: 10.1523/JNEUROSCI.20-19-07478.2000
Cortical feedback controls the frequency and synchrony of oscillations in the visual thalamus
Abstract
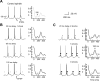
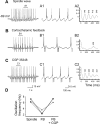
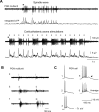
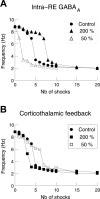
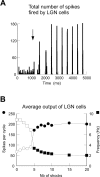
Thalamic circuits have an intrinsic capacity to generate state-dependent oscillations of different frequency and degrees of synchrony, but little is known of how synchronized oscillation is controlled in the intact brain or what function it may serve. The influence of cortical feedback was examined using slice preparations of the visual thalamus and computational models. Cortical feedback was mimicked by stimulating corticothalamic axons, triggered by the activity of relay neurons. This artificially coupled network had the capacity to self-organize and to generate qualitatively different rhythmical activities according to the strength of corticothalamic feedback stimuli. Weak feedback (one to three shocks at 100-150 Hz) phase-locked the spontaneous spindle oscillations (6-10 Hz) in geniculate and perigeniculate nuclei. However, strong feedback (four to eight shocks at 100-150 Hz) led to a more synchronized oscillation, slower in frequency (2-4 Hz) and dependent on GABA(B) receptors. This increase in synchrony was essentially attributable to a redistribution of the timing of action potential generation in lateral geniculate nucleus cells, resulting in an increased output of relay cells toward the cortex. Corticothalamic feedback is thus capable of inducing highly synchronous slow oscillations in physiologically intact thalamic circuits. This modulation may have implications for a better understanding of the descending control of thalamic nuclei by the cortex, and the genesis of pathological rhythmical activity, such as absence seizures.
Figures



References
-
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. - PubMed
-
- Ahlsen G, Grant K, Lindström S. Monosynaptic excitation of principal cells in the lateral geniculate nucleus by corticofugal fibers. Brain Res. 1982;234:454–458. - PubMed
-
- Andersen P, Andersson SA. Physiological basis of the alpha rhythm. Appelton Century Crofts; New York: 1968.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
