Seasonal population dynamics and interactions of competing bacteriophages and their host in the rhizosphere
- PMID: 11010859
- PMCID: PMC92285
- DOI: 10.1128/AEM.66.10.4193-4199.2000
Seasonal population dynamics and interactions of competing bacteriophages and their host in the rhizosphere
Abstract
We describe two prolonged bacteriophage blooms within sugar beet rhizospheres ensuing from an artificial increase in numbers of an indigenous soil bacterium. Further, we provide evidence of in situ competition between these phages. This is the first in situ demonstration of such microbial interactions in soil. To achieve this, sugar beet seeds were inoculated with Serratia liquefaciens CP6RS or its lysogen, CP6RS-ly-phi 1. These were sown, along with uninoculated seeds, in 36 field plots arranged in a randomized Latin square. The plots were then sampled regularly over 194 days, and the plants were assayed for the released bacteria and any infectious phages. Both the lysogen and nonlysogen forms of CP6RS survived equally well in situ, contradicting earlier work suggesting lysogens have a competitive disadvantage in nature. A Podoviridae phage, identified as phi CP6-4, flourished on the nonlysogen-inoculated plants in contrast to those plants inoculated with the lysogen. Conversely, the Siphoviridae phage phi CP6-1 (used to construct the released lysogen) was isolated abundantly from the lysogen-treated plants but almost never on the nonlysogen-inoculated plants. The uninoculated plants also harbored some phi CP6-1 phage up to day 137, yet hardly any phi CP6-4 phages were found, and this was consistent with previous years. We show that the different temporal and spatial distributions of these two physiologically distinct phages can be explained by application of optimal foraging theory to phage ecology. This is the first time that such in situ evidence has been provided in support of this theoretical model.
Figures

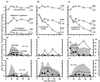

References
-
- Abedon S T. Selection for bacteriophage latent period length by bacterial density: a theoretical examination. Microb Ecol. 1989;18:79–88. - PubMed
-
- Adams M H. Bacteriophages. New York, N.Y: Interscience Publishers; 1959.
-
- Bergh O, Borcheim K Y, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

