A rhamnolipid biosurfactant reduces cadmium toxicity during naphthalene biodegradation
- PMID: 11010924
- PMCID: PMC92350
- DOI: 10.1128/AEM.66.10.4585-4588.2000
A rhamnolipid biosurfactant reduces cadmium toxicity during naphthalene biodegradation
Abstract
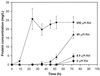
A model cocontaminated system was developed to determine whether a metal-complexing biosurfactant, rhamnolipid, could reduce metal toxicity to allow enhanced organic biodegradation by a Burkholderia sp. isolated from soil. Rhamnolipid eliminated cadmium toxicity when added at a 10-fold greater concentration than cadmium (890 microM), reduced toxicity when added at an equimolar concentration (89 microM), and had no effect at a 10-fold smaller concentration (8.9 microM). The mechanism by which rhamnolipid reduces metal toxicity may involve a combination of rhamnolipid complexation of cadmium and rhamnolipid interaction with the cell surface to alter cadmium uptake.
Figures


References
-
- Babich H, Stotzky G. Effect of cadmium on microbes in vitro and in vivo: influence of clay minerals. In: Babich H, Stotzky G, editors. Microbial ecology. Berlin, Germany: Springer-Verlag; 1978. pp. 412–415.
-
- Enger E D, Smith B F. Hazardous and toxic wastes. In: Enger E D, Smith B F, editors. Environmental science: the study of interrelationships. Dubuque, Iowa: Wm. C. Brown Publishers; 1992.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

