Decreased UDP-GlcNAc levels abrogate proliferation control in EMeg32-deficient cells
- PMID: 11013212
- PMCID: PMC302091
- DOI: 10.1093/emboj/19.19.5092
Decreased UDP-GlcNAc levels abrogate proliferation control in EMeg32-deficient cells
Abstract
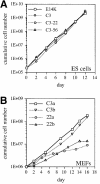
The hexosamine pathway provides UDP-N:-acetylhexosamine donor substrates used in cytosolic and Golgi-mediated glycosylation of proteins and for formation of glycosylphosphatidylinositol (GPI) anchors, which tether proteins to the outer plasma membrane. We have recently identified the murine glucosamine-6-phosphate (GlcN6P) acetyltransferase, EMeg32, as a developmentally regulated enzyme on the route to UDP-N:-acetylglucosamine (UDP-GlcNAc). Here we describe embryos and cells that have the EMeg32 gene inactivated by homologous recombination. Homozygous mutant embryos die at around embryonic day (E) 7.5 with a general proliferative delay of development. In vitro differentiated EMeg32(-/-) ES cells show reduced proliferation. Mouse embryonic fibroblasts (MEFs) deficient for EMeg32 exhibit defects in proliferation and adhesiveness, which could be complemented by stable re-expression of EMeg32 or by nutritional restoration of intracellular UDP-GlcNAc levels. Reduced UDP-GlcNAc levels predominantly translated into decreased O-GlcNAc modifications of cytosolic and nuclear proteins. Interestingly, growth-impaired EMeg32(-/-) MEFs withstand a number of apoptotic stimuli and express activated PKB/AKT. Thus, EMeg32-dependent UDP-GlcNAc levels influence cell cycle progression and susceptibility to apoptotic stimuli.
Figures













References
-
- Acharya U., Jacobs,R., Peters,J.M., Watson,N., Farquhar,M.G. and Malhotra,V. (1995) The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell, 82, 895–904. - PubMed
-
- Ayscough K. (1998) Use of latrunculin-A, an actin monomer-binding drug. Methods Enzymol., 298, 18–25. - PubMed
-
- Bamburg J.R., McGough,A. and Ono,S. (1999) Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol., 9, 364–370. - PubMed
-
- Boehmelt G., Fialka,I., Brothers,G., McGinley,M.D., Patterson,S.D., Mo,R., Hui,C.C., Huber,L.A., Mak,T.W. and Iscove,N.N. (2000) Cloning and characterization of the murine glucosamine-6-phosphate acetyltransferase EMeg32. J. Biol. Chem., 275, 12821–12832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

