Differential regulation of P2Y(11) receptor-mediated signalling to phospholipase C and adenylyl cyclase by protein kinase C in HL-60 promyelocytes
- PMID: 11015299
- PMCID: PMC1572341
- DOI: 10.1038/sj.bjp.0703581
Differential regulation of P2Y(11) receptor-mediated signalling to phospholipase C and adenylyl cyclase by protein kinase C in HL-60 promyelocytes
Abstract
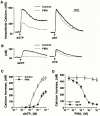
The regulatory mode of the P2Y(11) purinoceptor-mediated signalling cascades towards phospholipase C and adenylyl cyclase was studied in HL-60 promyelocytes. Treatment with the potent P2Y(11) receptor activator dATP evoked an elevated intracellular Ca(2+) concentration ([Ca(2+)](i)) and inositol 1,4,5-trisphosphate (IP(3)) production that was sustained for longer than 30 min. However, the dATP-induced responses were significantly inhibited by the activation of protein kinase C after a short exposure to phorbol 12-myristate 13-acetate (PMA). dATP also potently stimulated cyclic AMP production with half maximum effect seen at 23+/-7 microM dATP. In addition, a 5-min pretreatment with PMA enhanced the dATP-stimulated cyclic AMP accumulation. PMA potentiated the cyclic AMP production when adenylyl cyclase was activated directly by forskolin or indirectly by G protein activation after cholera toxin treatment. dATP also enhanced the forskolin-mediated cyclic AMP generation. Treatment of the cells with 10 microM U-73122, which almost completely blocked the dATP-stimulated IP(3) production and [Ca(2+)](i) rise, had no effect on cyclic AMP accumulation, while 10 microM 9-(tetrahydro-2-furyl)adenine (SQ 22536), which inhibited the adenylyl cyclase activation, did not effect the dATP-stimulated phosphoinositide turnover. Taken together, the results indicate that P2Y(11) receptor-mediated activation of phospholipase C and adenylyl cyclase occurs through independent pathways and is differentially regulated by protein kinase C in HL-60 cells.
Figures





Similar articles
-
Activation of the CD3/T cell receptor (TcR) complex or of protein kinase C potentiate adenylyl cyclase stimulation in a tumoral T cell line: involvement of two distinct intracellular pathways.Eur J Immunol. 1991 Nov;21(11):2877-82. doi: 10.1002/eji.1830211133. Eur J Immunol. 1991. PMID: 1657616
-
Sensitization of adenylyl cyclase by P2 purinergic and M5 muscarinic receptor agonists in L cells.Mol Pharmacol. 1991 Oct;40(4):539-46. Mol Pharmacol. 1991. PMID: 1921986
-
Involvement of protein kinase C in the UTP-mediated potentiation of cyclic AMP accumulation in mouse J774 macrophages.Br J Pharmacol. 1997 Aug;121(8):1749-57. doi: 10.1038/sj.bjp.0701300. Br J Pharmacol. 1997. PMID: 9283713 Free PMC article.
-
Advances in signalling by extracellular nucleotides. the role and transduction mechanisms of P2Y receptors.Cell Signal. 2000 Jun;12(6):351-60. doi: 10.1016/s0898-6568(00)00083-8. Cell Signal. 2000. PMID: 10889463 Review.
-
Mechanisms of phospholipase C activation: a comparison with the adenylate cyclase system.Biochimie. 1987 Apr;69(4):351-63. doi: 10.1016/0300-9084(87)90026-5. Biochimie. 1987. PMID: 3115315 Review.
Cited by
-
Differential coupling of the human P2Y(11) receptor to phospholipase C and adenylyl cyclase.Br J Pharmacol. 2001 Jan;132(1):318-26. doi: 10.1038/sj.bjp.0703788. Br J Pharmacol. 2001. PMID: 11156592 Free PMC article.
-
Regulation and organization of adenylyl cyclases and cAMP.Biochem J. 2003 Nov 1;375(Pt 3):517-29. doi: 10.1042/BJ20031061. Biochem J. 2003. PMID: 12940771 Free PMC article. Review.
-
A critical look at the function of the P2Y11 receptor.Purinergic Signal. 2016 Sep;12(3):427-37. doi: 10.1007/s11302-016-9514-7. Epub 2016 May 31. Purinergic Signal. 2016. PMID: 27246167 Free PMC article. Review.
-
Differentiation of hematopoietic stem cell and myeloid populations by ATP is modulated by cytokines.Cell Death Dis. 2011 Jun 2;2(6):e165. doi: 10.1038/cddis.2011.49. Cell Death Dis. 2011. PMID: 21633388 Free PMC article.
-
Caspase-3 and -9 are activated in human myeloid HL-60 cells by calcium signal.Mol Cell Biochem. 2010 Jan;333(1-2):151-7. doi: 10.1007/s11010-009-0215-1. Epub 2009 Jul 21. Mol Cell Biochem. 2010. PMID: 19626422
References
-
- ABBRACCHIO M.P., BURNSTOCK G. Purinoceptors: are there families of P2X and P2Y purinoceptors. Pharmacol. Ther. 1994;64:445–475. - PubMed
-
- ALI H., FISHER I., HARIBABU B., RICHARDSON R.M., SNYDERMAN R. Role of phospholipase Cβ3 phosphorylation in the desensitization of cellular responses to platelet-activating factor. J. Biol. Chem. 1997;272:11706–11709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

