Rapid quantification and differentiation of human polyomavirus DNA in undiluted urine from patients after bone marrow transplantation
- PMID: 11015385
- PMCID: PMC87458
- DOI: 10.1128/JCM.38.10.3689-3695.2000
Rapid quantification and differentiation of human polyomavirus DNA in undiluted urine from patients after bone marrow transplantation
Abstract
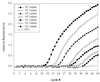
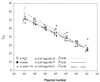
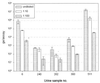
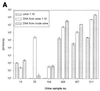
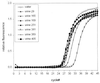
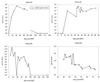
A combined PCR assay was developed for the detection and typing of human polyomavirus (huPoV) in clinical samples, consisting of (i) a qualitative seminested PCR assay (snPCR) to discriminate between huPoV BK and JC and (ii) a high-throughput, quantitative TaqMan PCR assay (TM-PCR) for the general detection of huPoV. The TM-PCR detects huPoV DNA in a linear range from 10(7) to 10(1) copies per assay. In reproducibility runs, the inter- and intra-assay variabilities were < or =60 and < or =50%, respectively. The snPCR assay uses a set of four primers for the same region of the BK and JC viral genomes. In the first round of amplification, two general primers were used; in the second round, one of these general primers and two additional, BK- or JC-specific primers were used simultaneously to produce amplicons of different sizes specific for BK virus (246 bp) and JC virus (199 bp), respectively. We tested different urine dilutions in order to determine the inhibitory effects of urine on PCR amplification. Furthermore, we compared the use of native urine with DNA purified by different preparation procedures. Our results show, that a 1:10 dilution of the urine led to complete reduction of the amplification inhibition found with 6% of undiluted urine samples. In a clinical study including 600 urine specimens, our assay turned out to be fast, cheap, and reliable in both qualitative and quantitative aspects.
Figures




References
-
- Arthur R R, Shah K V, Baust S J, Santos G W, Saral R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Engl J Med. 1986;315:230–234. - PubMed
-
- Arthur R R, Shah K V. Occurrence and significance of papovaviruses BK and JC in the urine. Prog Med Virol. 1989;36:42–61. - PubMed
-
- Azzi A, et al. Monitoring of polyomavirus BK viruria in bone marrow transplantation patients by DNA hybridization assay and by polymerase chain reaction: an approach to assess the relationship between BK viruria and hemorrhagic cystitis. Bone Marrow Transplant. 1994;14:235–240. - PubMed
-
- Azzi A, Zakrzewska K, Cesaro A, Fanci R, Bosi A. Monitoring of BKV viral load in urine of bone marrow transplantation patients and hemorrhagic cystitis. Acta Microbiol Immunol Hung. 1999;46:370–371.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

