Molecular and antigenic characterization of a highly evolved derivative of the type 2 oral poliovaccine strain isolated from sewage in Israel
- PMID: 11015392
- PMCID: PMC87465
- DOI: 10.1128/JCM.38.10.3729-3734.2000
Molecular and antigenic characterization of a highly evolved derivative of the type 2 oral poliovaccine strain isolated from sewage in Israel
Abstract
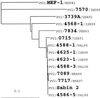
An unusual, highly diverged derivative of the Sabin type 2 oral poliovaccine (OPV) strain was recovered from environmental samples during routine screening for wild polioviruses. Virus was cultivated in L20B cells and then passaged on BGM cells at 40 degrees C (RCT [reproductive capacity at supraoptimal temperature]-positive marker) to select against most OPV strains. All but 1 of 25 RCT-positive OPV-derived environmental isolates were antigenically and genetically (>99.5% VP1 sequence match) similar to the respective Sabin strains. However, isolate PV2/4568-1/ISR98 (referred to below as 4568-1) escaped neutralization with Sabin 2-specific monoclonal antibodies and cross-adsorbed sera, and had multiple nucleotide substitutions (220 of 2,646; 8.3%) in the P1 capsid region. Fourteen of the 44 associated amino acid substitutions in the capsid mapped to neutralizing antigenic sites. Neutralizing titers in the sera of 50 Israeli children 15 years old were significantly lower to 4568-1 (geometric mean titer [GMT], 47) than to Sabin 2 (GMT, 162) or to the prototype wild strain, PV2/MEF-1/EGY42 (GMT, 108). Two key attenuating sites had also reverted in 4568-1 (A(481) to G in the 5' untranslated region and the VP1 amino acid I(143) to T), and the isolate was highly neurovirulent for transgenic mice expressing the poliovirus receptor (PVR-Tg21 mice). The extensive genetic divergence of 4568-1 from the parental Sabin 2 strain suggested that the virus had replicated in one or more people for approximately 6 years. The presence in the environment of a highly evolved, neurovirulent OPV-derived poliovirus in the absence of polio cases has important implications for strategies for the cessation of immunization with OPV following global polio eradication.
Figures


Similar articles
-
Oral poliovaccine: will it help eradicate polio or cause the next epidemic?Isr Med Assoc J. 2006 May;8(5):312-5. Isr Med Assoc J. 2006. PMID: 16805227
-
Genetic analysis and characterization of wild poliovirus type 1 during sustained transmission in a population with >95% vaccine coverage, Israel 2013.Clin Infect Dis. 2015 Apr 1;60(7):1057-64. doi: 10.1093/cid/ciu1136. Epub 2014 Dec 30. Clin Infect Dis. 2015. PMID: 25550350
-
Neurovirulent vaccine-derived polioviruses in sewage from highly immune populations.PLoS One. 2006 Dec 20;1(1):e69. doi: 10.1371/journal.pone.0000069. PLoS One. 2006. PMID: 17183700 Free PMC article.
-
Prevalence of vaccine-derived polioviruses in sewage and river water in South Africa.Water Res. 2005 Sep;39(14):3309-19. doi: 10.1016/j.watres.2005.05.027. Water Res. 2005. PMID: 15996707
-
Genomic modifications in Sabin vaccine strains isolated from vaccination-associated cases, healthy contacts and healthy vaccinees.Acta Virol. 1996 Jun;40(3):157-70. Acta Virol. 1996. PMID: 8891097 Review.
Cited by
-
Feasibility of quantitative environmental surveillance in poliovirus eradication strategies.Appl Environ Microbiol. 2012 Jun;78(11):3800-5. doi: 10.1128/AEM.07972-11. Epub 2012 Mar 23. Appl Environ Microbiol. 2012. PMID: 22447593 Free PMC article.
-
Metagenomic sequencing, molecular characterization, and Bayesian phylogenetics of imported type 2 vaccine-derived poliovirus, Spain, 2021.Front Cell Infect Microbiol. 2023 May 2;13:1168355. doi: 10.3389/fcimb.2023.1168355. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37201115 Free PMC article.
-
Vaccine-Derived Polioviruses and Children with Primary Immunodeficiency, Iran, 1995-2014.Emerg Infect Dis. 2016 Oct;22(10):1712-9. doi: 10.3201/eid2210.151071. Emerg Infect Dis. 2016. PMID: 27648512 Free PMC article.
-
Dynamics of Evolution of Poliovirus Neutralizing Antigenic Sites and Other Capsid Functional Domains during a Large and Prolonged Outbreak.J Virol. 2018 Apr 13;92(9):e01949-17. doi: 10.1128/JVI.01949-17. Print 2018 May 1. J Virol. 2018. PMID: 29444940 Free PMC article.
-
Antiviral activity of 3(2H)- and 6-chloro-3(2H)-isoflavenes against highly diverged, neurovirulent vaccine-derived, type2 poliovirus sewage isolates.PLoS One. 2011;6(5):e18360. doi: 10.1371/journal.pone.0018360. Epub 2011 May 25. PLoS One. 2011. PMID: 21904594 Free PMC article.
References
-
- Abraham R, Minor P, Dunn G, Modlin J F, Ogra P L. Shedding of virulent poliovirus revertants during immunization with oral poliovirus vaccine after prior immunization with inactivated polio vaccine. J Infect Dis. 1993;168:1105–1109. - PubMed
-
- Alexander J P, Jr, Gary H E, Jr, Pallansch M A. Duration of poliovirus excretion and its implications for acute flaccid paralysis surveillance: a review of the literature. J Infect Dis. 1997;175(Suppl. 1):S176–S182. - PubMed
-
- Centers for Disease Control and Prevention. Progress toward the global interruption of wild poliovirus type 2 transmission, 1999. Morb Mortal Wkly Rep. 1999;48:736–738. - PubMed
-
- Cochi S L, Sutter R W, Kew O M, Pallansch M A, Dowdle W R. A decision tree for stopping polio immunization. Technical Consultation on Global Eradication of Poliomyelitis. EPI/POLIO/TECH.97/WP18. Geneva, Switzerland: World Health Organization; 1997.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

