CD1b-mediated T cell recognition of a glycolipid antigen generated from mycobacterial lipid and host carbohydrate during infection
- PMID: 11015438
- PMCID: PMC2193317
- DOI: 10.1084/jem.192.7.965
CD1b-mediated T cell recognition of a glycolipid antigen generated from mycobacterial lipid and host carbohydrate during infection
Abstract
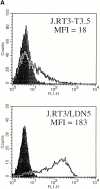
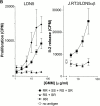
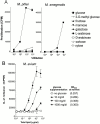
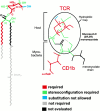
T cells recognize microbial glycolipids presented by CD1 proteins, but there is no information regarding the generation of natural glycolipid antigens within infected tissues. Therefore, we determined the molecular basis of CD1b-restricted T cell recognition of mycobacterial glycosylated mycolates, including those produced during tissue infection in vivo. Transfection of the T cell receptor (TCR) alpha and beta chains from a glucose monomycolate (GMM)-specific T cell line reconstituted GMM recognition in TCR-deficient T lymphoblastoma cells. This TCR-mediated response was highly specific for natural mycobacterial glucose-6-O-(2R, 3R) monomycolate, including the precise structure of the glucose moiety, the stereochemistry of the mycolate lipid, and the linkage between the carbohydrate and the lipid. Mycobacterial production of antigenic GMM absolutely required a nonmycobacterial source of glucose that could be supplied by adding glucose to media at concentrations found in mammalian tissues or by infecting tissue in vivo. These results indicate that mycobacteria synthesized antigenic GMM by coupling mycobacterial mycolates to host-derived glucose. Specific T cell recognition of an epitope formed by interaction of host and pathogen biosynthetic pathways provides a mechanism for immune response to those pathogenic mycobacteria that have productively infected tissues, as distinguished from ubiquitous, but innocuous, environmental mycobacteria.
Figures







References
-
- Sturgill-Koszycki S., Schlesinger P.H., Chakraborty P., Haddix P.L., Collins H.L., Fok A.K., Allen R.D., Gluck S.L., Heuser J., Russell D.G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. - PubMed
-
- Ferrari G., Langen H., Naito M., Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97:435–447. - PubMed
-
- Kaufmann S.H. Immunity to intracellular microbial pathogens. Immunol. Today. 1995;16:338–342. - PubMed
-
- North R.J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell. Immunol. 1973;7:166–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

