Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis
- PMID: 11015442
- PMCID: PMC2193315
- DOI: 10.1084/jem.192.7.1015
Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis
Abstract
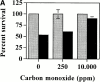
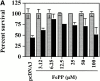
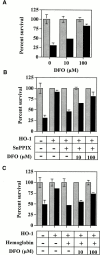
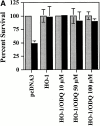
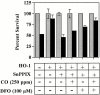
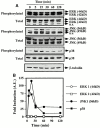
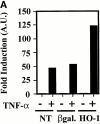
Heme oxygenase 1 (HO-1) inhibits apoptosis by regulating cellular prooxidant iron. We now show that there is an additional mechanism by which HO-1 inhibits apoptosis, namely by generating the gaseous molecule carbon monoxide (CO). Overexpression of HO-1, or induction of HO-1 expression by heme, protects endothelial cells (ECs) from apoptosis. When HO-1 enzymatic activity is blocked by tin protoporphyrin (SnPPIX) or the action of CO is inhibited by hemoglobin (Hb), HO-1 no longer prevents EC apoptosis while these reagents do not affect the antiapoptotic action of bcl-2. Exposure of ECs to exogenous CO, under inhibition of HO-1 activity by SnPPIX, substitutes HO-1 in preventing EC apoptosis. The mechanism of action of HO-1/CO is dependent on the activation of the p38 mitogen-activated protein kinase (MAPK) signaling transduction pathway. Expression of HO-1 or exposure of ECs to exogenous CO enhanced p38 MAPK activation by TNF-alpha. Specific inhibition of p38 MAPK activation by the pyridinyl imidazol SB203580 or through overexpression of a p38 MAPK dominant negative mutant abrogated the antiapoptotic effect of HO-1. Taken together, these data demonstrate that the antiapoptotic effect of HO-1 in ECs is mediated by CO and more specifically via the activation of p38 MAPK by CO.
Figures
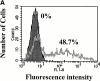



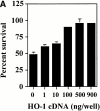

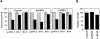
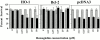







References
-
- Cines D.B., Pollak E.S., Buck C.A., Loscalzo J., Zimmerman G.A., McEver R.P., Pober J.S., Wick T.M., Konkle B.A., Schwartz B.S. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. - PubMed
-
- Hughes C.C., Savage C.O., Pober J.S. The endothelial cell as a regulator of T-cell function. Immunol. Rev. 1990;117:85–102. - PubMed
-
- Mantovani A., Bussolino F., Introna M. Cytokine regulation of endothelial cell functionfrom molecular level to the bedside. Immunol. Today. 1997;18:231–240. - PubMed
-
- Springer T.A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. - PubMed
-
- Soares M.P., Ferran C., Sato K., Takigami K., Anrather J., Lin Y., Bach F.H. Protective responses of endothelial cells. In: World Health Organization and Foundation IPSEN. V. Boulyjenkov, K. Berg, and Y. Christen,, editor. Genes and Resistance to Disease. Springer-Verlag; Heidelberg: 2000. pp. 91–104.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

