The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors
- PMID: 11016955
- PMCID: PMC17238
- DOI: 10.1073/pnas.200238297
The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors
Abstract
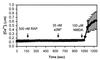
The low density lipoprotein receptor-related protein (LRP) is an endocytic receptor that is a member of the low density lipoprotein receptor family. We report that the LRP ligand, activated alpha(2)-macroglobulin (alpha(2)M*), induces robust calcium influx in cultured primary neurons, but not in nonneuronal LRP-containing cells in the same culture. The calcium influx is mediated through N-methyl-d-aspartate receptor channels, which explains the neuron specificity of the response. Microapplication of alpha(2)M* leads to a localized response at the site of application that dissipates rapidly, suggesting that the calcium signal is temporally and spatially discrete. Calcium influx to alpha(2)M* is blocked by the physiological LRP inhibitor, receptor-associated protein. Bivalent antibodies to the extracellular domain of LRP, but not Fab fragments of the same antibody, cause calcium influx, indicating that the response is specific to LRP and may require dimerization of the receptor. Thus, LRP is an endocytic receptor with a novel signaling role.
Figures





References
-
- Rebeck G W, Reiter J S, Strickland D K, Hyman B T. Neuron. 1993;11:575–580. - PubMed
-
- Strickland D K, Ashcom J D, Williams S, Burgess W H, Migliorini M, Argraves W S. J Biol Chem. 1990;265:17401–17404. - PubMed
-
- Williams S E, Ashcom J D, Argraves W S, Strickland D K. J Biol Chem. 1992;267:9035–9040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

