IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice
- PMID: 11016962
- PMCID: PMC17219
- DOI: 10.1073/pnas.200363097
IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice
Abstract
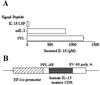
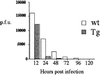
A transgenic (Tg) mouse expressing human IL-15 was generated to define the role of IL-15 in the normal immune response. Overexpression of IL-15 resulted in an increase of NK, CD44(hi)CD8 memory T cells, and gammadelta T cells. Additionally, we observed the emergence of a novel type of NK-T cells with CD8alphaalpha' expression. Due to the expansion and activation of NK cells, the IL-15Tg mouse showed enhanced innate immunity. In adaptive T cell immunity, the roles of IL-15 contrasted with those of IL-2. IL-15 inhibited IL-2-induced T cell death, which plays a role in the maintenance of peripheral self-tolerance. IL-15 thus seems to contribute to enhanced immune memory by selectively propagating memory T cells and by blocking T cell death mediated by IL-2.
Figures

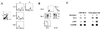
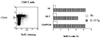

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

