Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability
- PMID: 11018017
- PMCID: PMC316970
- DOI: 10.1101/gad.836800
Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability
Abstract
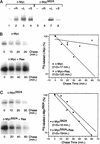
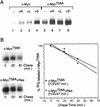
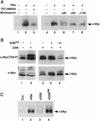
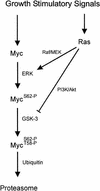
Our recent work has shown that activation of the Ras/Raf/ERK pathway extends the half-life of the Myc protein and thus enhances the accumulation of Myc activity. We have extended these observations by investigating two N-terminal phosphorylation sites in Myc, Thr 58 and Ser 62, which are known to be regulated by mitogen stimulation. We now show that the phosphorylation of these two residues is critical for determining the stability of Myc. Phosphorylation of Ser 62 is required for Ras-induced stabilization of Myc, likely mediated through the action of ERK. Conversely, phosphorylation of Thr 58, likely mediated by GSK-3 but dependent on the prior phosphorylation of Ser 62, is associated with degradation of Myc. Further analysis demonstrates that the Ras-dependent PI-3K pathway is also critical for controlling Myc protein accumulation, likely through the control of GSK-3 activity. These observations thus define a synergistic role for multiple Ras-mediated phosphorylation pathways in the control of Myc protein accumulation during the initial stage of cell proliferation.
Figures


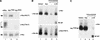

References
-
- Bhatia K, Huppi K, Spangler G, Siwarski D, Iyer R, Magrath I. Point mutations in the c-Myc transactivation domain are common in Burkitt's lymphoma and mouse plasmacytomas. Nat Genet. 1993;5:56–61. - PubMed
-
- Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. - PubMed
-
- Charron J, Malynn BA, Fisher P, Stewart V, Jeannotte L, Goff SP, Robertson EJ, Alt FW. Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes & Dev. 1992;6:2248–2257. - PubMed
-
- Clark HM, Yano T, Otsuki T, Jaffe ES, Shibata D, Raffeld M. Mutations in the coding region of c-MYC in AIDS-associated and other aggressive lymphomas. Cancer Res. 1994;54:3383–3386. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
