Antagonism between C/EBPbeta and FOG in eosinophil lineage commitment of multipotent hematopoietic progenitors
- PMID: 11018018
- PMCID: PMC316981
- DOI: 10.1101/gad.177200
Antagonism between C/EBPbeta and FOG in eosinophil lineage commitment of multipotent hematopoietic progenitors
Abstract
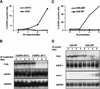
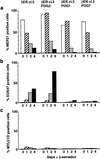
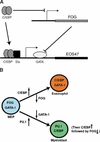
The commitment of multipotent cells to particular developmental pathways requires specific changes in their transcription factor complement to generate the patterns of gene expression characteristic of specialized cell types. We have studied the role of the GATA cofactor Friend of GATA (FOG) in the differentiation of avian multipotent hematopoietic progenitors. We found that multipotent cells express high levels of FOG mRNA, which were rapidly down-regulated upon their C/EBPbeta-mediated commitment to the eosinophil lineage. Expression of FOG in eosinophils led to a loss of eosinophil markers and the acquisition of a multipotent phenotype, and constitutive expression of FOG in multipotent progenitors blocked activation of eosinophil-specific gene expression by C/EBPbeta. Our results show that FOG is a repressor of the eosinophil lineage, and that C/EBP-mediated down-regulation of FOG is a critical step in eosinophil lineage commitment. Furthermore, our results indicate that maintenance of a multipotent state in hematopoiesis is achieved through cooperation between FOG and GATA-1. We present a model in which C/EBPbeta induces eosinophil differentiation by the coordinate direct activation of eosinophil-specific promoters and the removal of FOG, a promoter of multipotency as well as a repressor of eosinophil gene expression.
Figures

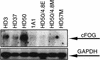


Similar articles
-
C/EBPbeta and GATA-1 synergistically regulate activity of the eosinophil granule major basic protein promoter: implication for C/EBPbeta activity in eosinophil gene expression.Blood. 1999 Aug 15;94(4):1429-39. Blood. 1999. PMID: 10438731
-
Distinct C/EBP functions are required for eosinophil lineage commitment and maturation.Genes Dev. 1998 Aug 1;12(15):2413-23. doi: 10.1101/gad.12.15.2413. Genes Dev. 1998. PMID: 9694805 Free PMC article.
-
Regulation of eosinophil-specific gene expression by a C/EBP-Ets complex and GATA-1.EMBO J. 1998 Jul 1;17(13):3669-80. doi: 10.1093/emboj/17.13.3669. EMBO J. 1998. PMID: 9649437 Free PMC article.
-
Hematopoietic development: a balancing act.Curr Opin Genet Dev. 2001 Oct;11(5):513-9. doi: 10.1016/s0959-437x(00)00226-4. Curr Opin Genet Dev. 2001. PMID: 11532392 Review.
-
[The trend of molecular biology study on eosinophils].Nihon Rinsho. 1993 Mar;51(3):741-6. Nihon Rinsho. 1993. PMID: 8492451 Review. Japanese.
Cited by
-
Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma.Allergy Asthma Immunol Res. 2012 Mar;4(2):68-79. doi: 10.4168/aair.2012.4.2.68. Epub 2011 Nov 25. Allergy Asthma Immunol Res. 2012. PMID: 22379601 Free PMC article.
-
A differential proteome screening system for post-translational modification-dependent transcription factor interactions.Nat Protoc. 2011 Mar;6(3):359-64. doi: 10.1038/nprot.2011.303. Epub 2011 Feb 24. Nat Protoc. 2011. PMID: 21372816
-
Cofactor-mediated restriction of GATA-1 chromatin occupancy coordinates lineage-specific gene expression.Mol Cell. 2012 Aug 24;47(4):608-21. doi: 10.1016/j.molcel.2012.05.051. Epub 2012 Jul 5. Mol Cell. 2012. PMID: 22771118 Free PMC article.
-
Distinct myeloid progenitor-differentiation pathways identified through single-cell RNA sequencing.Nat Immunol. 2016 Jun;17(6):666-676. doi: 10.1038/ni.3412. Epub 2016 Apr 4. Nat Immunol. 2016. PMID: 27043410 Free PMC article.
-
Hematopoiesis: an evolving paradigm for stem cell biology.Cell. 2008 Feb 22;132(4):631-44. doi: 10.1016/j.cell.2008.01.025. Cell. 2008. PMID: 18295580 Free PMC article.
References
-
- Beug H, Doederlein G, Freudenstein C, Graf T. Erythroblast cell lines transformed by a temperature sensitive mutant of avian erythroblastosis virus: A model system to study erythroid differentiation in vitro. J Cell Physiol Suppl. 1982;1:195–207. - PubMed
-
- Briegel K, Lim KC, Plank C, Beug H, Engel JD, Zenke M. Ectopic expression of a conditional GATA-2/estrogen receptor chimera arrests erythroid differentiation in a hormone-dependent manner. Genes & Dev. 1993;7:1097–1109. - PubMed
-
- Chomczynski P, Sacchi N. Single step isolation of RNA by acid guanidinium isothiocyanate method. Anal Biochem. 1987;162:156–159. - PubMed
-
- Crispino JD, Lodish MB, MacKay JP, Orkin SH. Use of altered specificity mutants to probe a specific protein-protein interaction in differentiation: The GATA-1:FOG complex. Mol Cell. 1999;3:219–228. - PubMed
-
- Deconinck AE, Mead PE, Tevosian SG, Crispino JD, Katz SG, Zon LI, Orkin SH. FOG acts as a repressor of red blood cell development in Xenopus. Development. 2000;127:2031–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
