Agrin isoforms with distinct amino termini: differential expression, localization, and function
- PMID: 11018052
- PMCID: PMC2189804
- DOI: 10.1083/jcb.151.1.41
Agrin isoforms with distinct amino termini: differential expression, localization, and function
Abstract
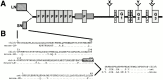
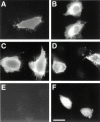
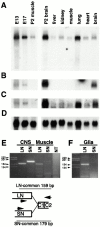
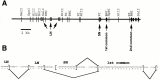
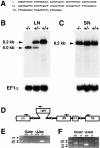
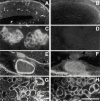
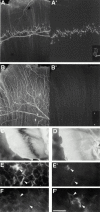
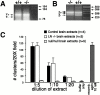
The proteoglycan agrin is required for postsynaptic differentiation at the skeletal neuromuscular junction, but is also associated with basal laminae in numerous other tissues, and with the surfaces of some neurons. Little is known about its roles at sites other than the neuromuscular junction, or about how its expression and subcellular localization are regulated in any tissue. Here we demonstrate that the murine agrin gene generates two proteins with different NH(2) termini, and present evidence that these isoforms differ in subcellular localization, tissue distribution, and function. The two isoforms share approximately 1,900 amino acids (aa) of common sequence following unique NH(2) termini of 49 or 150 aa; we therefore call them short NH(2)-terminal (SN) and long NH(2)-terminal (LN) isoforms. In the mouse genome, LN-specific exons are upstream of an SN-specific exon, which is in turn upstream of common exons. LN-agrin is expressed in both neural and nonneural tissues. In spinal cord it is expressed in discrete subsets of cells, including motoneurons. In contrast, SN-agrin is selectively expressed in the nervous system but is widely distributed in many neuronal cell types. Both isoforms are externalized from cells but LN-agrin assembles into basal laminae whereas SN-agrin remains cell associated. Differential expression of the two isoforms appears to be transcriptionally regulated, whereas the unique SN and LN sequences direct their distinct subcellular localizations. Insertion of a "gene trap" construct into the mouse genome between the LN and SN exons abolished expression of LN-agrin with no detectable effect on expression levels of SN-agrin or on SN-agrin bioactivity in vitro. Agrin protein was absent from all basal laminae in mice lacking LN-agrin transcripts. The formation of the neuromuscular junctions was as drastically impaired in these mutants as in mice lacking all forms of agrin. Thus, basal lamina-associated LN-agrin is required for neuromuscular synaptogenesis, whereas cell-associated SN-agrin may play distinct roles in the central nervous system.
Figures

References
-
- Barber A.J., Lieth E. Agrin accumulates in the brain microvascular basal lamina during development of the blood-brain barrier. Dev. Dyn. 1997;208:62–74. - PubMed
-
- Burgess R.W., Nguyen Q.T., Son Y.J., Lichtman J.W., Sanes J.R. Alternatively spliced isoforms of nerve- and muscle-derived agrintheir roles at the neuromuscular junction. Neuron. 1999;23:33–44. - PubMed
-
- Campanelli J.T., Hoch W., Rupp F., Kreiner T., Scheller R.H. Agrin mediates cell contact-induced acetylcholine receptor clustering. Cell. 1991;67:909–916. - PubMed
-
- Campanelli J.T., Gayer G.G., Scheller R.H. Alternative RNA splicing that determines agrin activity regulates binding to heparin and a-dystroglycan. Development. 1996;122:1663–1672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

