Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy
- PMID: 11018058
- PMCID: PMC2189812
- DOI: 10.1083/jcb.151.1.117
Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy
Abstract
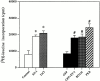
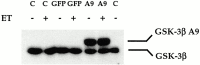
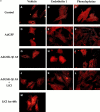
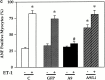
Hypertrophy is a basic cellular response to a variety of stressors and growth factors, and has been best characterized in myocytes. Pathologic hypertrophy of cardiac myocytes leads to heart failure, a major cause of death and disability in the developed world. Several cytosolic signaling pathways have been identified that transduce prohypertrophic signals, but to date, little work has focused on signaling pathways that might negatively regulate hypertrophy. Herein, we report that glycogen synthase kinase-3beta (GSK-3beta), a protein kinase previously implicated in processes as diverse as development and tumorigenesis, is inactivated by hypertrophic stimuli via a phosphoinositide 3-kinase-dependent protein kinase that phosphorylates GSK-3beta on ser 9. Using adenovirus-mediated gene transfer of GSK-3beta containing a ser 9 to alanine mutation, which prevents inactivation by hypertrophic stimuli, we demonstrate that inactivation of GSK-3beta is required for cardiomyocytes to undergo hypertrophy. Furthermore, our data suggest that GSK-3beta regulates the hypertrophic response, at least in part, by modulating the nuclear/cytoplasmic partitioning of a member of the nuclear factor of activated T cells family of transcription factors. The identification of GSK-3beta as a transducer of antihypertrophic signals suggests that novel therapeutic strategies to treat hypertrophic diseases of the heart could be designed that target components of the GSK-3 pathway.
Figures













References
-
- Avruch J. Insulin signal transduction through protein kinase cascades. Mol. Cell. Biochem. 1998;182:31–48. - PubMed
-
- Beals C.R., Clipstone N.A., Ho S.N., Crabtree G.R. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction Genes Dev. 11 1997. 824 834a - PubMed
-
- Beals C.R., Sheridan C.M., Turck C.W., Gardner P., Crabtree G.R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3 Science. 275 1997. 1930 1934b - PubMed
-
- Boyle W.J., Smeal T., Defize L.H., Angel P., Woodgett J.R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991;64:573–584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

