Microtubule and motor-dependent endocytic vesicle sorting in vitro
- PMID: 11018063
- PMCID: PMC2189808
- DOI: 10.1083/jcb.151.1.179
Microtubule and motor-dependent endocytic vesicle sorting in vitro
Abstract
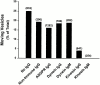
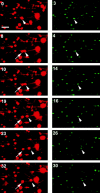
Endocytic vesicles undergo fission to sort ligand from receptor. Using quantitative immunofluorescence and video imaging, we provide the first in vitro reconstitution of receptor-ligand sorting in early endocytic vesicles derived from rat liver. We show that to undergo fission, presegregation vesicles must bind to microtubules (MTs) and move upon addition of ATP. Over 13% of motile vesicles elongate and are capable of fission. After fission, one vesicle continues to move, whereas the other remains stationary, resulting in their separation. On average, almost 90% receptor is found in one daughter vesicle, whereas ligand is enriched by approximately 300% with respect to receptor in the other daughter vesicle. Although studies performed on polarity marked MTs showed approximately equal plus and minus end-directed motility, immunofluorescence microscopy revealed that kinesins, but not dynein, were associated with these vesicles. Motility and fission were prevented by addition of 1 mM 5'-adenylylimido-diphosphate (AMP-PNP, an inhibitor of kinesins) or incubation with kinesin antibodies, but were unaffected by addition of 5 microM vanadate (a dynein inhibitor) or dynein antibodies. These studies indicate an essential role of kinesin-based MT motility in endocytic vesicle sorting, providing a system in which factors required for endocytic vesicle processing can be identified and characterized.
Figures






References
-
- Brady S.T. Molecular motors in the nervous system. Neuron. 1991;7:521–533. - PubMed
-
- Chang T.M., Chang C.H. Diacytosis of asialoglycoprotein in isolated hepatocytes is dependent on the structure of ligand and cellular distribution of the receptors. Biochim. Biophys. Acta. 1989;1014:229–234. - PubMed
-
- Forgac M. Structure, function and regulation of the vacuolar (H+)-ATPases. FEBS Lett. 1998;440:258–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

