Chronic alcohol ingestion induces osteoclastogenesis and bone loss through IL-6 in mice
- PMID: 11018077
- PMCID: PMC381425
- DOI: 10.1172/JCI10483
Chronic alcohol ingestion induces osteoclastogenesis and bone loss through IL-6 in mice
Abstract
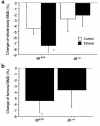
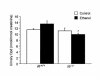
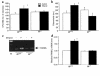
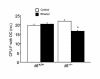
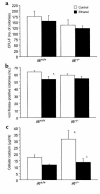
To investigate the role of IL-6 in alcohol-mediated osteoporosis, we measured a variety of bone remodeling parameters in wild-type (il6(+/+)) or IL-6 gene knockout (il6(-/-)) mice that were fed either control or ethanol liquid diets for 4 months. In the il6(+/+) mice, ethanol ingestion decreased bone mineral density, as determined by dual-energy densitometry; decreased cancellous bone volume and trabecular width and increased trabecular spacing and osteoclast surface, as determined by histomorphometry of the femur; increased urinary deoxypyridinolines, as determined by ELISA; and increased CFU-GM formation and osteoclastogenesis as determined ex vivo in bone marrow cell cultures. In contrast, ethanol ingestion did not alter any of these parameters in the il6(-/-) mice. Ethanol increased receptor activator of NF-kappaB ligand (RANKL) mRNA expression in the bone marrow of il6(+/+) but not il6(-/-) mice. Additionally, ethanol decreased several osteoblastic parameters including osteoblast perimeter and osteoblast culture calcium retention in both il6(+/+) and il6(-/-) mice. These findings demonstrate that ethanol induces bone loss through IL-6. Furthermore, they suggest that IL-6 achieves this effect by inducing RANKL and promoting CFU-GM formation and osteoclastogenesis.
Figures
Similar articles
-
Osteoprotegerin abrogates chronic alcohol ingestion-induced bone loss in mice.J Bone Miner Res. 2002 Jul;17(7):1256-63. doi: 10.1359/jbmr.2002.17.7.1256. J Bone Miner Res. 2002. PMID: 12096839
-
IL-1 plays an important role in the bone metabolism under physiological conditions.Int Immunol. 2010 Oct;22(10):805-16. doi: 10.1093/intimm/dxq431. Epub 2010 Aug 2. Int Immunol. 2010. PMID: 20679512
-
Ovariectomy-induced bone loss in TNFα and IL6 gene knockout mice is regulated by different mechanisms.J Mol Endocrinol. 2018 Apr;60(3):185-198. doi: 10.1530/JME-17-0218. Epub 2018 Jan 16. J Mol Endocrinol. 2018. PMID: 29339399
-
IL-6 is not required for parathyroid hormone stimulation of RANKL expression, osteoclast formation, and bone loss in mice.Am J Physiol Endocrinol Metab. 2005 Nov;289(5):E784-93. doi: 10.1152/ajpendo.00029.2005. Epub 2005 Jun 14. Am J Physiol Endocrinol Metab. 2005. PMID: 15956054
-
Impaired bone resorption in cathepsin K-deficient mice is partially compensated for by enhanced osteoclastogenesis and increased expression of other proteases via an increased RANKL/OPG ratio.Bone. 2005 Jan;36(1):159-72. doi: 10.1016/j.bone.2004.09.020. Epub 2004 Nov 24. Bone. 2005. PMID: 15664014
Cited by
-
Cytoprotective effect of American ginseng in a rat ethanol gastric ulcer model.Molecules. 2013 Dec 27;19(1):316-26. doi: 10.3390/molecules19010316. Molecules. 2013. PMID: 24378970 Free PMC article.
-
DNA damage drives accelerated bone aging via an NF-κB-dependent mechanism.J Bone Miner Res. 2013 May;28(5):1214-28. doi: 10.1002/jbmr.1851. J Bone Miner Res. 2013. PMID: 23281008 Free PMC article.
-
Alcohol affects the late differentiation of progenitor B cells.Alcohol Alcohol. 2011 Jan-Feb;46(1):26-32. doi: 10.1093/alcalc/agq076. Epub 2010 Nov 22. Alcohol Alcohol. 2011. PMID: 21098503 Free PMC article.
-
Diagnosis and Management of Cirrhosis-Related Osteoporosis.Biomed Res Int. 2016;2016:1423462. doi: 10.1155/2016/1423462. Epub 2016 Oct 20. Biomed Res Int. 2016. PMID: 27840821 Free PMC article. Review.
-
Iron-enriched diet contributes to early onset of osteoporotic phenotype in a mouse model of hereditary hemochromatosis.PLoS One. 2018 Nov 14;13(11):e0207441. doi: 10.1371/journal.pone.0207441. eCollection 2018. PLoS One. 2018. PMID: 30427936 Free PMC article.
References
-
- Seeman E, Melton J, O’Fallon M, Riggs BL. Risk factors for spinal osteoporosis in men. Am J Med. 1983;75:977–983. - PubMed
-
- Diamond T, Stiel D, Lunzer M, Wilkinson M, Posen S. Ethanol reduces bone formation and may cause osteoporosis. Am J Med. 1989;86:282–288. - PubMed
-
- Spencer H, Rubio N, Rubio E, Indreika M, Seitam A. Chronic alcoholism: frequently overlooked cause of osteoporosis in men. Am J Med. 1986;80:393–397. - PubMed
-
- Hannan MT, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

