Dominantly inherited hyperinsulinism caused by a mutation in the sulfonylurea receptor type 1
- PMID: 11018078
- PMCID: PMC381424
- DOI: 10.1172/JCI9804
Dominantly inherited hyperinsulinism caused by a mutation in the sulfonylurea receptor type 1
Abstract
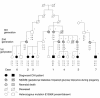
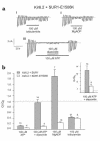
ATP-sensitive potassium channels play a major role in linking metabolic signals to the exocytosis of insulin in the pancreatic beta cell. These channels consist of two types of protein subunit: the sulfonylurea receptor SUR1 and the inward rectifying potassium channel Kir6.2. Mutations in the genes encoding these proteins are the most common cause of congenital hyperinsulinism (CHI). Since 1973, we have followed up 38 pediatric CHI patients in Finland. We reported previously that a loss-of-function mutation in SUR1 (V187D) is responsible for CHI of the most severe cases. We have now identified a missense mutation, E1506K, within the second nucleotide binding fold of SUR1, found heterozygous in seven related patients with CHI and in their mothers. All patients have a mild form of CHI that usually can be managed by long-term diazoxide treatment. This clinical finding is in agreement with the results of heterologous coexpression studies of recombinant Kir6.2 and SUR1 carrying the E1506K mutation. Mutant K(ATP) channels were insensitive to metabolic inhibition, but a partial response to diazoxide was retained. Five of the six mothers, two of whom suffered from hypoglycemia in infancy, have developed gestational or permanent diabetes. Linkage and haplotype analysis supported a dominant pattern of inheritance in a large pedigree. In conclusion, we describe the first dominantly inherited SUR1 mutation that causes CHI in early life and predisposes to later insulin deficiency.
Figures
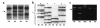



References
-
- Aynsley-Green A. Nesidioblastosis of the pancreas in infancy. Dev Med Child Neurol. 1981;23:372–379. - PubMed
-
- Permutt MA, Nestorowicz A, Glaser B. Familial hyperinsulinism: an inherited disorder of spontaneous hypoglycemia in neonates and infants. Diabetes Rev. 1996;4:347–355.
-
- Meissner T, Brune W, Mayatepek E. Persistent hyperinsulinaemic hypoglycaemia of infancy: therapy, clinical outcome and mutational analysis. Eur J Pediatr. 1997;156:754–757. - PubMed
-
- Thomas PM, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

