Effect of intracellular pH on spontaneous Ca2+ sparks in rat ventricular myocytes
- PMID: 11018103
- PMCID: PMC2270124
- DOI: 10.1111/j.1469-7793.2000.00025.x
Effect of intracellular pH on spontaneous Ca2+ sparks in rat ventricular myocytes
Abstract
1. A fall of intracellular pH (pHi) typically depresses cardiac contractility. Among the many mechanisms underlying this depression, an inhibitory effect of acidosis upon the sarcoplasmic reticulum (SR) Ca2+ release channel has been predicted, but not so far demonstrated in the intact cardiac myocyte. In the present work, pHi was manipulated experimentally while confocal imaging was used to record spontaneous 'Ca2+ sparks' (local SR Ca2+ release events) in rat isolated myocytes loaded with the fluorescent Ca2+ indicator fluo-3. In other experiments, whole cell (global) pHi or [Ca2+]i was measured by microfluorimetry (using, respectively, intracellular carboxy SNARF-1 and indo-1). 2. Reducing pHi (i) increased whole cell intracellular [Ca2+] transients induced either electrically or by addition of caffeine, whereas (ii) it decreased spontaneous Ca2+ spark frequency. Conversely, raising pHi increased spontaneous Ca2+ spark frequency. 3. Blocking sarcolemmal Ca2+ influx with 10 mM Ni2+, or reducing external pH by 1.0 unit, had no effect on the pHi-dependent changes in spontaneous Ca2+ spark frequency. 4. Decreasing pHi over the range 7.78-7.20, decreased Ca2+ spark frequency exponentially as a function of pHi, with frequency declining by approximately 33 % for a 0.2 unit fall in pHi. In contrast, over the same pHi range, Ca2+ spark amplitude was unaffected. Intracellular acidosis produced a slight slowing of Ca2+ spark relaxation. 5. The results indicate that, in the intact myocyte, a reduced pHi decreases the probability of opening of the SR Ca2+ release channel. This phenomenon may contribute to the negative inotropic effects of acidosis.
Figures
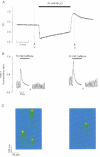


 ), and in NT solution of pH 6.4 (
), and in NT solution of pH 6.4 ( ). Decrease in pHi (±
). Decrease in pHi (±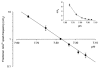
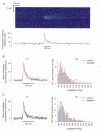
References
-
- Berlin JR, Cannell MB, Lederer WJ. Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circulation Research. 1989;65:115–126. - PubMed
-
- Buckler KJ, Vaughan-Jones RD. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflügers Archiv. 1990;417:234–239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

