Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones
- PMID: 11018104
- PMCID: PMC2270119
- DOI: 10.1111/j.1469-7793.2000.00039.x
Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones
Abstract
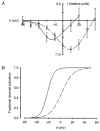
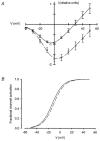
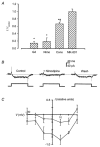
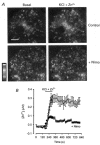
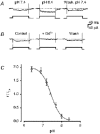
1. Mammalian neuronal voltage-gated Ca2+ channels have been implicated as potential mediators of membrane permeability to Zn2+. We tested directly whether voltage-gated Ca2+ channels can flux Zn2+ in whole-cell voltage-clamp recordings from cultured murine cortical neurones. 2. In the presence of extracellular Zn2+ and no Na+, K+, or other divalent cations, a small, non-inactivating, voltage-gated inward current was observed exhibiting a current-voltage relationship characteristic of high-voltage activated (HVA) Ca2+ channels. Inward current was detectable at Zn2+ levels as low as 50 microM, and both the amplitude and voltage sensitivity of the current depended upon Zn2+ concentration. This Zn2+ current was sensitive to blockade by Gd3+ and nimodipine and, to a lesser extent, by omega-conotoxin GVIA. 3. Zn2+ could permeate Ca2+ channels in the presence of Ca2+ and other physiological cations. Inward currents recorded with 2 mM Ca2+ were attenuated by Zn2+ (IC50 = 210 microM), and currents recorded with Zn2+ were unaffected by up to equimolar Ca2+ concentrations. Furthermore, the Zn2+-selective fluorescent dye Newport Green revealed a depolarisation-activated, nimodipine-sensitive Zn2+ influx into cortical neurones that were bathed in a physiological extracellular solution plus 300 microM ZnCl2. 4. Surprisingly, while lowering extracellular pH suppressed HVA Ca2+ currents, Zn2+ current amplitude was affected oppositely, varying inversely with pH with an apparent pK of 7.4. The acidity-induced enhancement of Zn2+ current was associated with a positive shift in reversal potential but no change in the kinetics or voltage sensitivity of channel activation. 5. These results provide evidence that L- and N-type voltage-gated Ca2+ channels can mediate Zn2+ entry into cortical neurones and that this entry may be enhanced by extracellular acidity.
Figures




References
-
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. - PubMed
-
- Atar D, Backx PH, Appel MM, Gao WD, Marban E. Excitation-transcription coupling mediated by zinc influx through voltage-dependent calcium channels. Journal of Biological Chemistry. 1995;270:2473–2477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

