Exogenous Ca2+-ATPase isoform effects on Ca2+ transients of embryonic chicken and neonatal rat cardiac myocytes
- PMID: 11018105
- PMCID: PMC2270107
- DOI: 10.1111/j.1469-7793.2000.00053.x
Exogenous Ca2+-ATPase isoform effects on Ca2+ transients of embryonic chicken and neonatal rat cardiac myocytes
Abstract
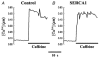
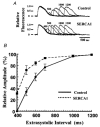
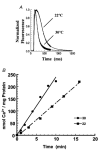
1. Sarco-endoplasmic reticulum Ca2+-ATPase from fast skeletal (SERCA1) or cardiac muscle (SERCA2a) was expressed in embryonic chicken and neonatal rat cardiac myocytes by adenovirus vectors, with c-myc tags on both constructs to compare expression and distinguish exogenous from endogenous SERCA2a in myocytes. 2. Expression of the two isoforms was similar (approximately 3-fold higher than endogenous SERCA). However, SERCA1 activity was 2-fold greater than SERCA2a activity, due to intrinsic differences in turnover rates. Activation of both exogenous SERCA isoforms by Ca2+ was displaced to slightly lower [Ca2+], suggesting that the overexpressed isoforms were independent of phospholamban. In fact, phospholamban and calsequestrin expression were unchanged. 3. Decay time constants of cytosolic Ca2+ transients from cells overexpressing SERCA1 were reduced by 30-40 % and half-widths by 10-15 % compared to controls. SERCA2a overexpression produced much less acceleration of transients in chick than in rat, and less acceleration than SERCA1 overexpression in either species. There was no significant change in resting [Ca2+], peak amplitudes, or in the amount of Ca2+ releasable by caffeine from overexpression of either SERCA isoform. However, the amplitudes of the transients increased with SERCA1 overexpression when pacing frequency limited refilling of the sarcoplasmic reticulum. 4. It is concluded that total SERCA transport velocity has a primary effect on the decay phase of transients. Transport velocity is affected by SERCA isoform turnover rate, temperature, and/or SERCA copy number.
Figures




Similar articles
-
Comparison of SERCA1 and SERCA2a expressed in COS-1 cells and cardiac myocytes.Am J Physiol. 1999 Dec;277(6):H2381-91. doi: 10.1152/ajpheart.1999.277.6.H2381. Am J Physiol. 1999. PMID: 10600859
-
Tight control of exogenous SERCA expression is required to obtain acceleration of calcium transients with minimal cytotoxic effects in cardiac myocytes.Circ Res. 2001 Mar 2;88(4):415-21. doi: 10.1161/01.res.88.4.415. Circ Res. 2001. PMID: 11230109
-
Cell-specific expression of SERCA, the exogenous Ca2+ transport ATPase, in cardiac myocytes.Am J Physiol Cell Physiol. 2004 Mar;286(3):C556-64. doi: 10.1152/ajpcell.00328.2003. Epub 2003 Oct 30. Am J Physiol Cell Physiol. 2004. PMID: 14592812
-
Mechanism of thyroid-hormone regulated expression of the SERCA genes in skeletal muscle: implications for thermogenesis.Biosci Rep. 2001 Apr;21(2):139-54. doi: 10.1023/a:1013692023449. Biosci Rep. 2001. PMID: 11725863 Review.
-
The regulation of sarco(endo)plasmic reticulum calcium-ATPases (SERCA).Can J Physiol Pharmacol. 2015 Oct;93(10):843-54. doi: 10.1139/cjpp-2014-0463. Epub 2015 Jan 19. Can J Physiol Pharmacol. 2015. PMID: 25730320 Review.
Cited by
-
Limited functional and metabolic improvements in hypertrophic and healthy rat heart overexpressing the skeletal muscle isoform of SERCA1 by adenoviral gene transfer in vivo.Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2483-94. doi: 10.1152/ajpheart.01023.2008. Epub 2008 Oct 24. Am J Physiol Heart Circ Physiol. 2008. PMID: 18952713 Free PMC article.
-
The Ca2+ ATPase of cardiac sarcoplasmic reticulum: Physiological role and relevance to diseases.Biochem Biophys Res Commun. 2008 Apr 25;369(1):182-7. doi: 10.1016/j.bbrc.2007.11.161. Epub 2007 Dec 7. Biochem Biophys Res Commun. 2008. PMID: 18068669 Free PMC article. Review.
-
SERCA1 expression enhances the metabolic efficiency of improved contractility in post-ischemic heart.J Mol Cell Cardiol. 2009 Nov;47(5):614-21. doi: 10.1016/j.yjmcc.2009.08.031. Epub 2009 Sep 8. J Mol Cell Cardiol. 2009. PMID: 19744494 Free PMC article.
-
Sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) gene silencing and remodeling of the Ca2+ signaling mechanism in cardiac myocytes.Proc Natl Acad Sci U S A. 2004 Nov 23;101(47):16683-8. doi: 10.1073/pnas.0407537101. Epub 2004 Nov 16. Proc Natl Acad Sci U S A. 2004. PMID: 15546997 Free PMC article.
-
Adenoviral SERCA1 overexpression triggers an apoptotic response in cultured neonatal but not in adult rat cardiomyocytes.Mol Cell Biochem. 2004 Dec;267(1-2):123-32. doi: 10.1023/b:mcbi.0000049361.89265.2b. Mol Cell Biochem. 2004. PMID: 15663193
References
-
- Anger M, Samuel JL, Marotte F, Wuytack F, Rappaport L, Lompre AM. In situ mRNA distribution of sarco(endo)plasmic reticulum Ca(2+)-ATPase isoforms during ontogeny in the rat. Journal of Molecular and Cellular Cardiology. 1994;26:539–550. - PubMed
-
- Baker DL, Hashimoto K, Grupp IL, Ji Y, Reed T, Loukianov E, Grupp G, Bhagwhat A, Hoit B, Walsh R, Marban E, Periasamy M. Targeted overexpression of the sarcoplasmic reticulum Ca2+-ATPase increases cardiac contractility in transgenic mouse hearts. Circulation Research. 1998;83:1205–1214. - PubMed
-
- Bers DM. Ryanodine and the calcium content of cardiac SR assessed by caffeine and rapid cooling contractures. American Journal of Physiology. 1987;253:C408–415. - PubMed
-
- Bokkala S, el Daher SS, Kakkar VV, Wuytack F, Authi KS. Localization and identification of Ca2+ATPases in highly purified human platelet plasma and intracellular membranes. Evidence that the monoclonal antibody PL/IM 430 recognizes the SERCA 3 Ca2+ATPase in human platelets. Biochemical Journal. 1995;306:837–842. - PMC - PubMed
-
- Brandl CJ, Green NM, Korczak B, MacLennan DH. Two Ca2+-ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986;44:597–607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

