Voltage-gated transient outward currents in neurons with different firing patterns in rat superior colliculus
- PMID: 11018108
- PMCID: PMC2270113
- DOI: 10.1111/j.1469-7793.2000.00091.x
Voltage-gated transient outward currents in neurons with different firing patterns in rat superior colliculus
Abstract
1. We investigated the electrophysiological properties of transient outward currents (TOCs) in neurons with different firing patterns, regular-spiking, fast-spiking and late-spiking neurons, in the intermediate layer (SGI) of the superior colliculus using the whole-cell patch clamp technique in slice preparations obtained from young rats (post-natal days 17-22). 2. Analysis of inactivation kinetics and normalized amplitude revealed that TOCs in regular-and fast-spiking neurons had fast inactivation kinetics (decay time constants (mean +/- s.e.m.) of 13.8 +/- 1.5 and 11.4 +/- 1.2 ms, respectively) and low current densities (36.6 +/- 3.3 and 32.1 +/- 4. 9 pA pF-1, respectively). TOCs in late-spiking neurons, on the other hand, displayed a wide range of both inactivation kinetics (36.7 +/- 2.4 ms, with a range from 11.3 to 147.8 ms) and current density (54. 0 +/- 2.9 pA pF-1, with a range from 9.8 to 131.2 pA pF-1). 3. In regular-, fast- and late-spiking neurons having TOCs with slow time constants (> 50 ms, class II late-spiking neurons), the TOCs were sensitive to 4-aminopyridine (4-AP), with IC50 values of 2.9, 2.4 and 1.2 mM, respectively. In late-spiking neurons having TOCs with fast decay time constants (< 30 ms, class I late-spiking neurons), the TOCs were composed of at least two 4-AP-sensitive components (IC50 values of 0.2 microM and 3.6 mM). 4. Class I late-spiking neurons displayed non-inactivating outward currents which were highly sensitive to 4-AP. They changed their firing patterns to the regular-spiking mode, not only in response to low concentrations of 4-AP (< 50 microM), but also in response to dendrotoxin (200 nM), suggesting that non-inactivating outward currents contribute to the late-spiking property. However, the components of TOCs which were highly sensitive to 4-AP were also sensitive to dendrotoxin. These results suggest that both or either of the two currents contribute to the late-spiking property of class I late-spiking neurons. 5. Although class II late-spiking neurons also displayed non-inactivating outward currents, the late-spiking property was not abolished by low concentrations of 4-AP and dendrotoxin. They changed to a regular firing pattern in response to a high concentration of 4-AP (5 mM), suggesting that TOCs contribute to late-spiking property of class II late-spiking neurons. 6. The results suggest that TOCs with different properties contribute to the different firing patterns of SGI neurons.
Figures

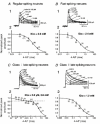

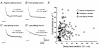
 , late-spiking neurons.
, late-spiking neurons.



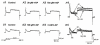
Similar articles
-
Layer I neurons of rat neocortex. I. Action potential and repetitive firing properties.J Neurophysiol. 1996 Aug;76(2):651-67. doi: 10.1152/jn.1996.76.2.651. J Neurophysiol. 1996. PMID: 8871189
-
Electrophysiological and morphological properties of neurons in the rat superior colliculus. I. Neurons in the intermediate layer.J Neurophysiol. 1999 Aug;82(2):754-67. doi: 10.1152/jn.1999.82.2.754. J Neurophysiol. 1999. PMID: 10444674
-
Potassium currents and membrane excitability of neurons in the rat's dorsal nucleus of the lateral lemniscus.J Neurophysiol. 1996 Aug;76(2):1121-32. doi: 10.1152/jn.1996.76.2.1121. J Neurophysiol. 1996. PMID: 8871225
-
Layer I neurons of the rat neocortex. II. Voltage-dependent outward currents.J Neurophysiol. 1996 Aug;76(2):668-82. doi: 10.1152/jn.1996.76.2.668. J Neurophysiol. 1996. PMID: 8871190
-
Role of voltage-gated K+ currents in mediating the regular-spiking phenotype of callosal-projecting rat visual cortical neurons.J Neurophysiol. 1997 Nov;78(5):2321-35. doi: 10.1152/jn.1997.78.5.2321. J Neurophysiol. 1997. PMID: 9356385
Cited by
-
Pyramidal neurons in the superficial layers of rat retrosplenial cortex exhibit a late-spiking firing property.Brain Struct Funct. 2013 Jan;218(1):239-54. doi: 10.1007/s00429-012-0398-1. Epub 2012 Mar 1. Brain Struct Funct. 2013. PMID: 22383041 Free PMC article.
-
Local excitatory network and NMDA receptor activation generate a synchronous and bursting command from the superior colliculus.J Neurosci. 2003 Jul 2;23(13):5854-64. doi: 10.1523/JNEUROSCI.23-13-05854.2003. J Neurosci. 2003. PMID: 12843290 Free PMC article.
-
Modulatory mechanisms and multiple functions of somatodendritic A-type K (+) channel auxiliary subunits.Front Cell Neurosci. 2014 Mar 27;8:82. doi: 10.3389/fncel.2014.00082. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24723849 Free PMC article. Review.
-
Dipeptidyl peptidase-like protein 6 is required for normal electrophysiological properties of cerebellar granule cells.J Neurosci. 2010 Jun 23;30(25):8551-65. doi: 10.1523/JNEUROSCI.5489-09.2010. J Neurosci. 2010. PMID: 20573902 Free PMC article.
-
Opposing effects of spinal nerve ligation on calcium-activated potassium currents in axotomized and adjacent mammalian primary afferent neurons.Brain Res. 2007 Feb 9;1132(1):84-99. doi: 10.1016/j.brainres.2006.11.055. Epub 2006 Dec 20. Brain Res. 2007. PMID: 17184741 Free PMC article.
References
-
- Andreasen M, Hablitz JJ. Kinetic properties of a transient outward current in rat neocortical neurons. Journal of Neurophysiology. 1992;68:1133–1142. - PubMed
-
- Baxter DA, Byrne JH. Ionic conductance mechanisms contributing to the electrophysiological properties of neurons. Current Opinion in Neurobiology. 1991;1:105–112. - PubMed
-
- Butler DM, Ono JK, Chang T, McCaman RE, Barish ME. Mouse brain potassium channel beta1 subunit mRNA: cloning and distribution during development. Journal of Neurobiology. 1998;34:135–150. - PubMed
-
- Chandy KG, Gutman GA. Voltage-gated K+ channel genes. In: North RA, editor. Handbook of Receptors and Channels: Ligand- and Voltage-gated Ion Channels. Boca Ralton, FL, USA: CRC Press; 1995. pp. 1–79.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

