Relationship between the time course of the afterhyperpolarization and discharge variability in cat spinal motoneurones
- PMID: 11018112
- PMCID: PMC2270116
- DOI: 10.1111/j.1469-7793.2000.t01-1-00131.x
Relationship between the time course of the afterhyperpolarization and discharge variability in cat spinal motoneurones
Abstract
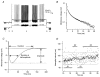
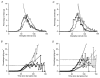
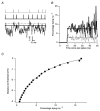
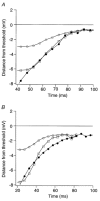
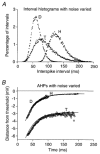
1. We elicited repetitive discharges in cat spinal motoneurones by injecting noisy current waveforms through a microelectrode to study the relationship between the time course of the motoneurone's afterhyperpolarization (AHP) and the variability in its spike discharge. Interspike interval histograms were used to estimate the interval death rate, which is a measure of the instantaneous probability of spike occurrence as a function of the time since the preceding spike. It had been previously proposed that the death rate can be used to estimate the AHP trajectory. We tested the accuracy of this estimate by comparing the AHP trajectory predicted from discharge statistics to the measured AHP trajectory of the motoneurone. 2. The discharge statistics of noise-driven cat motoneurones shared a number of features with those previously reported for voluntarily activated human motoneurones. At low discharge rates, the interspike interval histograms were often positively skewed with an exponential tail. The standard deviation of the interspike intervals increased with the mean interval, and the plots of standard deviation versus the mean interspike interval generally showed an upward bend, the onset of which was related to the motoneurone's AHP duration. 3. The AHP trajectories predicted from the interval death rates were generally smaller in amplitude (i.e. less hyperpolarized) than the measured AHP trajectories. This discrepancy may result from the fact that spike threshold varies during the interspike interval, so that the distance to threshold at a given time depends upon both the membrane trajectory and the spike threshold trajectory. Nonetheless, since the interval death rate is likely to reflect the instantaneous distance to threshold during the interspike interval, it provides a functionally relevant measure of fluctuations in motoneurone excitability during repetitive discharge.
Figures







Similar articles
-
Contributions of the input signal and prior activation history to the discharge behaviour of rat motoneurones.J Physiol. 2005 Feb 1;562(Pt 3):707-24. doi: 10.1113/jphysiol.2004.069039. Epub 2004 Dec 20. J Physiol. 2005. PMID: 15611038 Free PMC article.
-
Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise.J Physiol. 1996 Apr 15;492 ( Pt 2)(Pt 2):597-628. doi: 10.1113/jphysiol.1996.sp021332. J Physiol. 1996. PMID: 9019553 Free PMC article.
-
What can be learned about motoneurone properties from studying firing patterns?Adv Exp Med Biol. 2002;508:199-205. doi: 10.1007/978-1-4615-0713-0_24. Adv Exp Med Biol. 2002. PMID: 12171112
-
Changes in motoneurone electrical properties following axotomy.J Physiol. 1979 Aug;293:197-215. doi: 10.1113/jphysiol.1979.sp012885. J Physiol. 1979. PMID: 501587 Free PMC article.
-
Historical reflections on the afterhyperpolarization--firing rate relation of vertebrate spinal neurons.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007 Feb;193(2):145-58. doi: 10.1007/s00359-006-0198-2. Epub 2006 Dec 28. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007. PMID: 17192827 Review.
Cited by
-
Glutamate modulates the firing rate in oculomotor nucleus motoneurons as a function of the recruitment threshold current.J Physiol. 2012 Jul 1;590(13):3113-27. doi: 10.1113/jphysiol.2011.226985. Epub 2012 May 8. J Physiol. 2012. PMID: 22570384 Free PMC article.
-
Age-related change in duration of afterhyperpolarization of human motoneurones.J Physiol. 2007 Dec 1;585(Pt 2):483-90. doi: 10.1113/jphysiol.2007.142356. Epub 2007 Oct 11. J Physiol. 2007. PMID: 17932149 Free PMC article.
-
Contributions of the input signal and prior activation history to the discharge behaviour of rat motoneurones.J Physiol. 2005 Feb 1;562(Pt 3):707-24. doi: 10.1113/jphysiol.2004.069039. Epub 2004 Dec 20. J Physiol. 2005. PMID: 15611038 Free PMC article.
-
Influence of afferent synaptic innervation on the discharge variability of cat abducens motoneurones.J Physiol. 2002 May 15;541(Pt 1):283-99. doi: 10.1113/jphysiol.2001.013405. J Physiol. 2002. PMID: 12015436 Free PMC article.
-
Variability of quadriceps femoris motor neuron discharge and muscle force in human aging.Exp Brain Res. 2007 May;179(2):219-33. doi: 10.1007/s00221-006-0785-z. Epub 2006 Nov 30. Exp Brain Res. 2007. PMID: 17136528
References
-
- Baldissera F, Gustafsson B. Afterhyperpolarization time course in lumbar motoneurones of the cat. Acta Physiologica Scandinavica. 1974;91:512–527. - PubMed
-
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 3–53.
-
- Binder MD, Robinson FR, Powers RK. Distribution of effective synaptic currents in cat triceps surae motoneurons. VI. Contralateral pyramidal tract. Journal of Neurophysiology. 1998;80:5241–5248. - PubMed
-
- Calvin WH. Three modes of repetitive firing and the role of threshold time course between spikes. Brain Research. 1974;69:341–346. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

