Isoform-specific and exercise intensity-dependent activation of 5'-AMP-activated protein kinase in human skeletal muscle
- PMID: 11018120
- PMCID: PMC2270117
- DOI: 10.1111/j.1469-7793.2000.t01-1-00221.x
Isoform-specific and exercise intensity-dependent activation of 5'-AMP-activated protein kinase in human skeletal muscle
Abstract
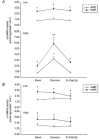
1. 5'-AMP-activated protein kinase (AMPK) has been suggested to play a key role in the regulation of metabolism in skeletal muscle. AMPK is activated in treadmill-exercised and electrically stimulated rodent muscles. Whether AMPK is activated during exercise in humans is unknown. 2. We investigated the degree of activation and deactivation of alpha-isoforms of AMPK during and after exercise. Healthy human subjects performed bicycle exercise on two separate occasions at either a low ( approximately 50% maximum rate of O2 uptake (VO2,max) for 90 min) or a high ( approximately 75% VO2,max for 60 min) intensity. Biopsies from the vastus lateralis muscle were obtained before and immediately after exercise, and after 3 h of recovery. 3. We observed a 3- to 4-fold activation of the alpha2-AMPK isoform immediately after high intensity exercise, whereas no activation was observed after low intensity exercise. The activation of alpha2-AMPK was totally reversed 3 h after exercise. In contrast, alpha1-AMPK was not activated during either of the two exercise trials. 4. The in vitro AMP dependency of alpha2-AMPK was significantly greater than that of alpha1-AMPK ( approximately 3- vs. approximately 2-fold). 5. We conclude that in humans activation of alpha2-AMPK during exercise is dependent upon exercise intensity. The stable activation of alpha2-AMPK, presumably due to the activation of an upstream AMPK kinase, is compatible with a role for this kinase complex in the regulation of skeletal muscle metabolism during exercise, whereas the lack of stable alpha1-AMPK activation makes this kinase complex a less likely candidate.
Figures
Comment in
-
How to avoid running on empty.J Physiol. 2000 Oct 1;528 Pt 1(Pt 1):3. doi: 10.1111/j.1469-7793.2000.t01-1-00003.x. J Physiol. 2000. PMID: 11018100 Free PMC article. No abstract available.
Similar articles
-
AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise.Diabetes. 2001 May;50(5):921-7. doi: 10.2337/diabetes.50.5.921. Diabetes. 2001. PMID: 11334434
-
Oral glucose ingestion attenuates exercise-induced activation of 5'-AMP-activated protein kinase in human skeletal muscle.Biochem Biophys Res Commun. 2006 Apr 14;342(3):949-55. doi: 10.1016/j.bbrc.2006.02.057. Biochem Biophys Res Commun. 2006. PMID: 16598851 Clinical Trial.
-
Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle.FASEB J. 2005 Jul;19(9):1146-8. doi: 10.1096/fj.04-3144fje. Epub 2005 May 5. FASEB J. 2005. PMID: 15878932
-
AMP-activated protein kinase regulation and action in skeletal muscle during exercise.Biochem Soc Trans. 2003 Feb;31(Pt 1):191-5. doi: 10.1042/bst0310191. Biochem Soc Trans. 2003. PMID: 12546683 Review.
-
Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models.Biochem Soc Trans. 2003 Feb;31(Pt 1):216-9. doi: 10.1042/bst0310216. Biochem Soc Trans. 2003. PMID: 12546688 Review.
Cited by
-
The nuclear receptor PPARβ/δ programs muscle glucose metabolism in cooperation with AMPK and MEF2.Genes Dev. 2011 Dec 15;25(24):2619-30. doi: 10.1101/gad.178434.111. Epub 2011 Dec 1. Genes Dev. 2011. PMID: 22135324 Free PMC article.
-
Respiratory issues in patients with multiple sclerosis as a risk factor during SARS-CoV-2 infection: a potential role for exercise.Mol Cell Biochem. 2023 Jul;478(7):1533-1559. doi: 10.1007/s11010-022-04610-1. Epub 2022 Nov 21. Mol Cell Biochem. 2023. PMID: 36411399 Free PMC article. Review.
-
Effects of Feeding Time on Markers of Muscle Metabolic Flexibility Following Acute Aerobic Exercise in Trained Mice Undergoing Time Restricted Feeding.Nutrients. 2021 May 19;13(5):1717. doi: 10.3390/nu13051717. Nutrients. 2021. PMID: 34069449 Free PMC article.
-
5' adenosine monophosphate-activated protein kinase, metabolism and exercise.Sports Med. 2004;34(2):91-103. doi: 10.2165/00007256-200434020-00003. Sports Med. 2004. PMID: 14965188 Review.
-
Stress signaling in the heart by AMP-activated protein kinase.Curr Hypertens Rep. 2006 Dec;8(6):446-50. doi: 10.1007/s11906-006-0021-z. Curr Hypertens Rep. 2006. PMID: 17087854 Review.
References
-
- Dean DJ, Daugaard JR, Young ME, Saha A, Vavvas D, Asp S, Kiens B, Kim K-H, Witters LA, Richter EA, Ruderman NB. Exercise disminishes the activity of acetyl CoA carboxylase in human muscle. Diabetes. 2000;49:1295–1300. - PubMed
-
- Derave W, Hua AI, Ihleman J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. - PubMed
-
- Gao G, Widmer J, Stapleton D, Teh T, Cox T, Kemp BE, Witters LA. Catalytic subunits of the porcine and rat 5′-AMP-activated protein kinase are members of the SNF1 protein kinase family. Biochimica et Biophysica Acta. 1995;1266:73–82. - PubMed
-
- Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annual Reviews of Biochemistry. 1998;67:821–855. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

