p75(NTR) mediates neurotrophin-induced apoptosis of vascular smooth muscle cells
- PMID: 11021829
- PMCID: PMC1850174
- DOI: 10.1016/S0002-9440(10)64640-8
p75(NTR) mediates neurotrophin-induced apoptosis of vascular smooth muscle cells
Abstract
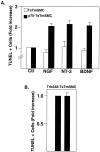
The development of atherosclerotic lesions results from aberrant cell migration, proliferation, and extracellular matrix production. In advanced lesions, however, cellular apoptosis, leading to lesion remodeling, predominates. During lesion formation, the neurotrophins and the neurotrophin receptor tyrosine kinases, trks B and C, are induced and mediate smooth muscle cell migration. Here we demonstrate that a second neurotrophin receptor, p75(NTR), is expressed by established human atherosclerotic lesions and late lesions that develop after balloon injury of the rat thoracic aorta. The p75(NTR), a member of the tumor necrosis factor/FAS receptor family, can modulate trk receptor function as well as initiate cell death when expressed in cells of the nervous system that lack kinase-active trk receptors. p75(NTR) expression colocalizes to neointimal cells, which express smooth muscle cell alpha-actin and are expressed by cultured human endarterectomy-derived cells (HEDC). Areas of the plaque expressing p75(NTR) demonstrate increased TUNEL positivity, and HEDC undergo apoptosis in response to the neurotrophins. Finally, neurotrophins also induced apoptosis of a smooth muscle cell line genetically manipulated to express p75(NTR), but lacking trk receptor expression. These studies identify the regulated expression of neurotrophins and p75(NTR) as an inducer of smooth muscle cell apoptosis in atherosclerotic lesions.
Figures








References
-
- Chao MV, Hempstead BL: p75 and trk: a two-receptor system. Trends Neurosci 1995, 18:321-326 - PubMed
-
- Donovan MJ, Miranda RC, Kraemer R, Mccaffrey TA, Tessarollo L, Mahadeo D, Sharif S, Kaplan DR, Tsoulfas P, Parada L, Toran-Allerand D, Hajjar DP, Hempstead BL: Neurotrophin and neurotrophin receptors in vascular smooth muscle cells: regulation of expression in response to injury. Am J Pathol 1995, 147:309-324 - PMC - PubMed
-
- Klein R, Lamballe F, Bryant S, Barbacid M: The trkB tyrosine protein kinase is a receptor for neurotrophin 4. Neuron 1992, 8:947-956 - PubMed
-
- Kraemer R, Ngyuen H, March KL, Hempstead BL: NGF activates similar intracellular signaling pathways in vascular smooth muscle cells as PDGF-BB but elicits different biological respones. Arterioscler Thromb Vasc Biol 1999, 19:1041-1050 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

