Localization of apoptotic macrophages at the site of plaque rupture in sudden coronary death
- PMID: 11021830
- PMCID: PMC1850160
- DOI: 10.1016/S0002-9440(10)64641-X
Localization of apoptotic macrophages at the site of plaque rupture in sudden coronary death
Abstract
Although apoptosis is a well-recognized phenomenon in chronic atherosclerotic disease, its role in sudden coronary death, in particular, acute plaque rupture is unknown. Culprit lesions from 40 cases of sudden coronary death were evaluated. Cases were divided into two mechanisms of death: ruptured plaques with acute thrombosis (n = 25) and stable plaques with and without healed myocardial infarction (n = 15). Apoptotic cells were identified by staining of fragmented DNA and confirmed in select cases by gold conjugate labeling combined with ultrastructural analysis. Additional studies were performed to examine the expression and activation of two inducers of apoptosis, caspases-1 and -3. Ruptured plaques showed extensive macrophage infiltration of the fibrous cap, in particular at rupture sites contrary to stable lesions, which contained fewer inflammatory cells. Among the culprit lesions, the overall incidence of apoptosis in fibrous caps was significantly greater in ruptured plaques (P < 0.001) and was predominantly localized to the CD68-positive macrophages. Furthermore, apoptosis at plaque rupture sites was more frequent than in areas of intact fibrous cap (P = 0. 028). Plaque rupture sites demonstrated a strong immunoreactivity to caspase-1 within the apoptotic macrophages; staining for caspase-3 was weak. Immunoblot analysis of ruptured plaques demonstrated caspase-1 up-regulation and the presence of its active p20 subunit whereas stable lesions showed only the precursor; nonatherosclerotic control segments were negative for both precursor and active enzyme. These findings demonstrate extensive apoptosis of macrophages limited to the site of plaque rupture. The proteolytic cleavage of caspase-1 in ruptured plaques suggests activation of this apoptotic precursor. Whether macrophage apoptosis is essential to acute plaque rupture or is a response to the rupture itself remains to be determined.
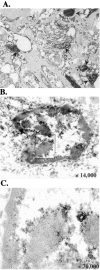
Figures





References
-
- Farb A, Burke AP, Tang AL, Liang Y, Mannan P, Smialek J, Virmani R: Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation 1996, 93:1354-1363 - PubMed
-
- Davies MJ: Anatomic features in victims of sudden coronary death. Coronary artery pathology. Circulation 1992, 85:19-24 - PubMed
-
- Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GR, Lee RT: Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol 1996, 7:330-335 - PubMed
-
- Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R: Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med 1997, 336:1276-1282 - PubMed
-
- Richardson PD, Davies MJ, Born GV: Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet 1989, 2:941-944 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

