CD18 and ICAM-1-dependent corneal neovascularization and inflammation after limbal injury
- PMID: 11021832
- PMCID: PMC1850165
- DOI: 10.1016/S0002-9440(10)64643-3
CD18 and ICAM-1-dependent corneal neovascularization and inflammation after limbal injury
Abstract
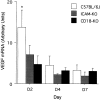
Extensive limbal injury is a leading cause of irreversible blindness. The destruction of corneal limbal stem cells often results in corneal neovascularization and an optically inferior epithelium. Previous work has shown that the neovascularization after limbal injury is vascular endothelial growth factor (VEGF)-dependent, with much of the VEGF emanating from the inflammatory cells that invade the cornea. Using a relevant mouse model of limbal injury, we examined the role of CD18 and intercellular adhesion molecule-1 (ICAM-1) in limbal injury-induced neovascularization. The results show that CD18- and ICAM-1-deficient mice developed 35% (n = 5, P = 0.003) and 36% (n = 5, P = 0.002) less neovascularization than strain-specific normal controls, respectively. The corneal neutrophil counts were similarly reduced by 51% (n = 5, P < 0.003) and 46% (n = 5, P < 0.006), respectively. When VEGF mRNA levels were analyzed, they were reduced by 66% (n = 3, P = 0.004) and 48% (n = 3, P = 0.024), respectively. Taken together, these data identify CD-18 and ICAM-1 as mediators of the inflammatory and VEGF-dependent corneal neovascularization that follows limbal injury. The targeting of CD18 and ICAM-1 may prove useful in the treatment of inflammation-associated neovascularization in the cornea and elsewhere.
Figures



References
-
- Tsai RJ, Sun TT, Tseng SC: Comparison of limbal and conjunctival autograft transplantation in corneal surface reconstruction in rabbits. Ophthalmology 1990, 97:446-455 - PubMed
-
- Huang AJW, Watson BD, Hernandez E, Tseng SCG: Induction of conjunctival transdifferentiation on vascularized corneas by photothrombotic occlusion of corneal neovascularization. Ophthalmology 1988, 95:228-235 - PubMed
-
- Amano S, Rohan R, Kuroki M, Tolentino M, Adamis AP: Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci 1998, 39:18-22 - PubMed
-
- Wilson RW, Ballantyne CM, Smith CW, Montgomery C, Bradley A, O’Brien WE, Beaudet AL: Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol 1993, 151:1571-1578 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

