Rubella virus replication and links to teratogenicity
- PMID: 11023958
- PMCID: PMC88950
- DOI: 10.1128/CMR.13.4.571
Rubella virus replication and links to teratogenicity
Abstract
Rubella virus (RV) is the causative agent of the disease known more popularly as German measles. Rubella is predominantly a childhood disease and is endemic throughout the world. Natural infections of rubella occur only in humans and are generally mild. Complications of rubella infection, most commonly polyarthralgia in adult women, do exist; occasionally more serious sequelae occur. However, the primary public health concern of RV infection is its teratogenicity. RV infection of women during the first trimester of pregnancy can induce a spectrum of congenital defects in the newborn, known as congenital rubella syndrome (CRS). The development of vaccines and implementation of vaccination strategies have substantially reduced the incidence of disease and in turn of CRS in developed countries. The pathway whereby RV infection leads to teratogenesis has not been elucidated, but the cytopathology in infected fetal tissues suggests necrosis and/or apoptosis as well as inhibition of cell division of critical precursor cells involved in organogenesis. In cell culture, a number of unusual features of RV replication have been observed, including mitochondrial abnormalities, and disruption of the cytoskeleton; these manifestations are most probably linked and play some role in RV teratogenesis. Further understanding of the mechanism of RV teratogenesis will be brought about by the investigation of RV replication and virus-host interactions.
Figures

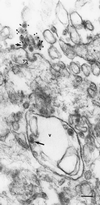
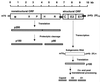
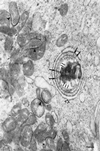

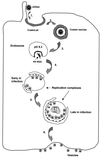
References
-
- Abe T, Nakada T, Hatanka H, Tajima M, Hiraiwa M, Ushijima H. Myoclonus in a case of suspected progressive rubella panencephalitis. Arch Neurol. 1983;40:98–100. - PubMed
-
- Acheson N H, Tamm I. Replication of Semliki Forest virus: an electron microscope study. Virology. 1967;32:128–143. - PubMed
-
- Atreya C D, Lee N S, Forng R-Y, Höfmann J, Washington G, Marti G, Nakhasi H L. The rubella virus putative replicase interacts with the retinoblastoma tumor suppressor protein. Virus Genes. 1998;16:177–183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

