Pathogenic roles for fungal melanins
- PMID: 11023965
- PMCID: PMC88958
- DOI: 10.1128/CMR.13.4.708
Pathogenic roles for fungal melanins
Abstract
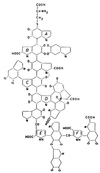
Melanins represent virulence factors for several pathogenic fungi; the number of examples is growing. Thus, albino mutants of several genera (in one case, mutated precisely in the melanizing enzyme) exhibit decreased virulence in mice. We consider the phenomenon in relation to known chemical properties of melanin, beginning with biosynthesis from ortho-hydroquinone precursors which, when oxidized enzymatically to quinones, polymerize spontaneously to melanin. It follows that melanizing intermediates are cross-linking reagents; melanization stabilizes the external cell wall against hydrolysis and is thought to determine semipermeability in the osmotic ram (the appressorium) of certain plant pathogens. Polymeric melanins undergo reversible oxidation-reduction reactions between cell wall-penetrating quinone and hydroquinone oxidation states and thus represent polymeric redox buffers; using strong oxidants, it is possible to titrate the melanin on living cells and thereby demonstrate protection conferred by melanin in several species. The amount of buffering per cell approximately neutralizes the amount of oxidant generated by a single macrophage. Moreover, the intermediate oxidation state, the semiquinone, is a very stable free radical and is thought to trap unpaired electrons. We have suggested that the oxidation state of external melanin may be regulated by external Fe(II). An independent hypothesis holds that in Cryptococcus neoformans, an important function of the melanizing enzyme (apart from melanization) is the oxidation of Fe(II) to Fe(III), thereby forestalling generation of the harmful hydroxyl radical from H(2)O(2). Thus, problems in fungal pathogenesis have led to evolving hypotheses regarding melanin functioning.
Figures




References
-
- Aver'yanov A A, Lapikova V P, Petelina G G, Dzhavakhiya V G. Increased sensitivity of pigment mutants of Pyricularia oryzae to toxic excretions of rice leaves. Fiziol Rast (Moscow) 1989;36:1088–1095.
-
- Bechinger C, Giebel K-F, Schnell M, Leiderer P, Deising H B, Bastmeyer M. Optical measurements of invasive forces exerted by appressoria of a plant pathogenic fungus. Science. 1999;285:1896–1899. - PubMed
-
- Bell A A, Wheeler M H. Biosynthesis and functions of fungal melanins. Annu Rev Phytopathol. 1986;24:411–451.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

