Discordance between bovine leukemia virus tax immortalization in vitro and oncogenicity in vivo
- PMID: 11024116
- PMCID: PMC102026
- DOI: 10.1128/jvi.74.21.9895-9902.2000
Discordance between bovine leukemia virus tax immortalization in vitro and oncogenicity in vivo
Abstract
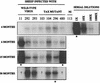
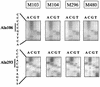
Bovine leukemia virus (BLV) Tax protein, a transcriptional activator of viral expression, is essential for viral replication in vivo. Tax is believed to be involved in leukemogenesis because of its second function, immortalization of primary cells in vitro. These activities of Tax can be dissociated on the basis of point mutations within specific regions of the protein. For example, mutation of the phosphorylation sites at serines 106 and 293 abrogates immortalization potential in vitro but maintains transcriptional activity. This type of mutant is thus particularly useful for unraveling the role of Tax immortalization activity during leukemogenesis independently of viral replication. In this report, we describe the biological properties of BLV recombinant proviruses mutated in the Tax phosphorylation sites (BLVTax106+293). Titration of the proviral loads by semiquantitative PCR revealed that the BLV mutants propagated at wild-type levels in vivo. Furthermore, two animals (sheep 480 and 296) infected with BLVTax106+293 developed leukemia or lymphosarcoma after 16 and 36 months, respectively. These periods of time are within the normal range of latencies preceding the onset of pathogenesis induced by wild-type viruses. The phenotype of the mutant-infected cells was characteristic of a B lymphocyte (immunoglobulin M positive) expressing CD11b and CD5 (except at the final stage for the latter marker), a pattern that is typical of wild-type virus-infected target cells. Interestingly, the transformed B lymphocytes from sheep 480 also coexpressed the CD8 marker, a phenotype rarely observed in tumor biopsies from chronic lymphocytic leukemia patients. Finally, direct sequencing of the tax gene demonstrated that the leukemic cells did not harbor revertant proviruses. We conclude that viruses expressing a Tax mutant unable to transform primary cells in culture are still pathogenic in the sheep animal model. Our data thus provide a clear example of the discordant conclusions that can be drawn from in vitro immortalization assays and in vivo experiments. These observations could be of interest for other systems, such as the related human T-cell leukemia virus type 1, which currently lack animal models allowing the study of the leukemogenic process.
Figures


Similar articles
-
Phosphorylation of bovine leukemia virus Tax protein is required for in vitro transformation but not for transactivation.Oncogene. 1998 Apr 30;16(17):2165-76. doi: 10.1038/sj.onc.1201765. Oncogene. 1998. PMID: 9619825
-
In vivo rescue of a silent tax-deficient bovine leukemia virus from a tumor-derived ovine B-cell line by recombination with a retrovirally transduced wild-type tax gene.J Virol. 1999 Feb;73(2):1054-65. doi: 10.1128/JVI.73.2.1054-1065.1999. J Virol. 1999. PMID: 9882306 Free PMC article.
-
Estimation of bovine leukemia virus (BLV) proviral load harbored by lymphocyte subpopulations in BLV-infected cattle at the subclinical stage of enzootic bovine leucosis using BLV-CoCoMo-qPCR.BMC Vet Res. 2013 May 4;9:95. doi: 10.1186/1746-6148-9-95. BMC Vet Res. 2013. PMID: 23641811 Free PMC article.
-
Genetic determinants of bovine leukemia virus pathogenesis.AIDS Res Hum Retroviruses. 2000 Nov 1;16(16):1787-95. doi: 10.1089/08892220050193326. AIDS Res Hum Retroviruses. 2000. PMID: 11080828 Review.
-
Cell dynamics and immune response to BLV infection: a unifying model.Front Biosci. 2007 Jan 1;12:1520-31. doi: 10.2741/2165. Front Biosci. 2007. PMID: 17127399 Review.
Cited by
-
Analysis of Nucleotide Sequence of Tax, miRNA and LTR of Bovine Leukemia Virus in Cattle with Different Levels of Persistent Lymphocytosis in Russia.Pathogens. 2021 Feb 20;10(2):246. doi: 10.3390/pathogens10020246. Pathogens. 2021. PMID: 33672613 Free PMC article.
-
BLV: lessons on vaccine development.Retrovirology. 2019 Oct 7;16(1):26. doi: 10.1186/s12977-019-0488-8. Retrovirology. 2019. PMID: 31590667 Free PMC article. Review.
-
Mechanisms of leukemogenesis induced by bovine leukemia virus: prospects for novel anti-retroviral therapies in human.Retrovirology. 2007 Mar 16;4:18. doi: 10.1186/1742-4690-4-18. Retrovirology. 2007. PMID: 17362524 Free PMC article. Review.
-
Increased cell proliferation, but not reduced cell death, induces lymphocytosis in bovine leukemia virus-infected sheep.Proc Natl Acad Sci U S A. 2002 Jul 23;99(15):10048-53. doi: 10.1073/pnas.142100999. Epub 2002 Jul 15. Proc Natl Acad Sci U S A. 2002. PMID: 12119390 Free PMC article.
-
A mutant form of the tax protein of bovine leukemia virus (BLV), with enhanced transactivation activity, increases expression and propagation of BLV in vitro but not in vivo.J Virol. 2003 Feb;77(3):1894-903. doi: 10.1128/jvi.77.3.1894-1903.2003. J Virol. 2003. PMID: 12525624 Free PMC article.
References
-
- Akagi T, Ono H, Nyunoya H, Shimotohno K. Characterization of peripheral blood T-lymphocytes transduced with HTLV-I Tax mutants with different trans-activating phenotypes. Oncogene. 1997;14:2071–2078. - PubMed
-
- Akashi K, Shibuya T, Nakamura M, Oogami A, Harada M, Niho Y. Large granular lymphocytic leukaemia with a mixed T-cell/B-cell phenotype. Br J Haematol. 1998;100:291–294. - PubMed
-
- Birkebak T A, Palmer G H, Davis W C, Knowles D P, McElwain T F. Association of GP51 expression and persistent CD5+ B-lymphocyte expansion with lymphomagenesis in bovine leukemia virus infected sheep. Leukemia. 1994;8:1890–1899. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

