Incoming human cytomegalovirus pp65 (UL83) contained in apoptotic infected fibroblasts is cross-presented to CD8(+) T cells by dendritic cells
- PMID: 11024130
- PMCID: PMC102040
- DOI: 10.1128/jvi.74.21.10018-10024.2000
Incoming human cytomegalovirus pp65 (UL83) contained in apoptotic infected fibroblasts is cross-presented to CD8(+) T cells by dendritic cells
Abstract
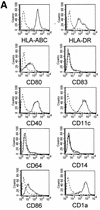
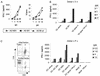
Human cytomegalovirus (HCMV) infection is well controlled mainly by cytotoxic CD8(+) T lymphocytes (CTL) directed against the matrix protein pp65 despite the numerous immune escape mechanisms developed by the virus. Dendritic cells (DCs) are key antigen-presenting cells for the generation of an immune response which have the capacity to acquire antigens via endocytosis of apoptotic cells and thus present peptides to major histocompatibility complex class I-restricted T cells. We examined whether this mechanism could contribute to the activation of anti-pp65 CTL. In this study, we show that infection by HCMV AD169 induced sensitization of MRC5 fibroblasts to tumor necrosis factor alpha-mediated apoptosis very early after virus inoculation and that pp65 contained in apoptotic cells came from the delivery of the matrix protein into the cell. We observed that immature DCs derived from peripheral monocytes were not permissive to HCMV AD169 infection but were able to internalize pp65-positive apoptotic infected MRC5 cells. We then demonstrated that following exposure to these apoptotic bodies, DCs could activate HLA-A2- or HLA-B35-restricted anti-pp65 CTL, suggesting that they acquired and processed properly fibroblast-derived pp65. Together, our data suggest that cross-presentation of incoming pp65 contained in apoptotic cells may provide a quick and efficient way to prime anti-HCMV CD8(+) T cells.
Figures





References
-
- Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. - PubMed
-
- Bell D, Young J, Banchereau J. Dendritic cells. Adv Immunol. 1999;72:255–324. - PubMed
-
- Britt W J, Alford C. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2493–2523.
-
- Chaudhuri A R, St. Jeor S, Maciejewski J P. Apoptosis induced by human cytomegalovirus infection can be enhanced by cytokines to limit the spread of virus. Exp Hematol. 1999;27:1194–1203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

