Deficiency of complement defense protein CD59 may contribute to neurodegeneration in Alzheimer's disease
- PMID: 11027207
- PMCID: PMC6772855
- DOI: 10.1523/JNEUROSCI.20-20-07505.2000
Deficiency of complement defense protein CD59 may contribute to neurodegeneration in Alzheimer's disease
Abstract
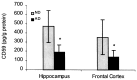
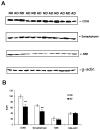
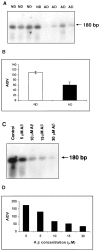
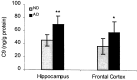
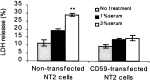
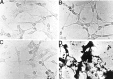
Complement defense 59 (CD59) is a cell surface glycophosphoinositol (GPI)-anchored protein that prevents complement membrane attack complex (MAC) assembly. Here, we present evidence from ELISA assays that CD59 protein levels are significantly decreased in the frontal cortex and hippocampus of Alzheimer's disease (AD) compared with nondemented elderly (ND) patients, whereas complement component 9, a final component to form MAC, is significantly increased. To further confirm the CD59 deficit, PI-specific phospholipase C (PIPLC) was used to cleave the CD59 GPI anchor at the cell surface in intact slices from AD and ND cortex. CD59 released by PIPLC cleavage was significantly reduced in AD compared with ND samples. By the use of a ribonuclease protection technique, amyloid beta-peptide was found to downregulate CD59 expression at the mRNA level, suggesting a partial explanation of CD59 deficits in the AD brain. To evaluate the pathophysiological significance of CD59 alterations in neurons, we exposed cultured NT2 cells, which normally underexpress CD59, and NT2 cells transfected to overexpress CD59 to homologous human serum. Lactic acid dehydrogenase assays revealed significant complement-induced cell lysis in CD59-underexpressing NT2 cells and significant protection from such lysis in CD59-overexpressing NT2 cells. Moreover, cells expressing normal levels of CD59 showed no evidence of MAC assembly or damage after exposure to homologous serum, whereas pretreatment of these cells with a CD59-neutralizing antibody resulted in MAC assembly at the cell surface and morphological damage. Taken together, these data suggest that CD59 deficits may play a role in the neuritic losses characteristic of AD.
Figures
References
-
- Eikelenboom P, Stam FC. Immunoglobulins and complement factors in senile plaques. Acta Neuropathol (Berl) 1982;57:239–242. - PubMed
-
- Itagaki S, Akiyama H, Saito H, McGeer PL. Ultrastructural localization of complement membrane attack complex (MAC)-like immunoreactivity in brains of patients with Alzheimer's disease. Brain Res. 1994;645:78–84. - PubMed
-
- Kim SH, Darney DF, Hammer CH, Shin ML. Nucleated cell killing by complement: effects of C5b-9 channel size and extracellular Ca2+ on the lytic process. J Immunol. 1987;138:1530–1536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
