Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A
- PMID: 11027216
- PMCID: PMC6772869
- DOI: 10.1523/JNEUROSCI.20-20-07571.2000
Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A
Abstract
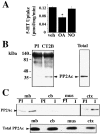
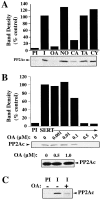
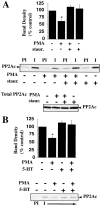
Presynaptic transporter proteins regulate the clearance of extracellular biogenic amines after release and are important targets for multiple psychoactive agents, including amphetamines, cocaine, and antidepressant drugs. Recent studies reveal that dopamine (DA), norepinephrine (NE), and serotonin (5-HT) transporters (DAT, NET, and SERT, respectively) are rapidly regulated by direct or receptor-mediated activation of cellular kinases, particularly protein kinase C (PKC). With SERTs, PKC activation results in activity-dependent transporter phosphorylation and sequestration. Protein phosphatase 1/2A (PP1/PP2A) inhibitors, such as okadaic acid (OA) and calyculin A, also promote SERT phosphorylation and functional downregulation. How kinase, phosphatase, and transporter activities are linked mechanistically is unclear. In the present study, we found that okadaic acid-sensitive phosphatase activity is enriched in SERT immunoprecipitates from human SERT stably transfected cells. Moreover, blots of these immunoprecipitates reveal the presence of PP2A catalytic subunit (PP2Ac), findings replicated using brain preparations. Whole-cell treatments with okadaic acid or calyculin A diminished SERT/PP2Ac associations. Phorbol esters, which trigger SERT phosphorylation, also diminish SERT/PP2Ac associations, effects that can be blocked by PKC antagonists as well as the SERT substrate 5-HT. Similar transporter/PP2Ac complexes were also observed in coimmunoprecipitation studies with NETs and DATs. Our findings provide evidence for the existence of regulated heteromeric assemblies involving biogenic amine transporters and PP2A and suggest that the dynamic stability of these complexes may govern transporter phosphorylation and sequestration.
Figures


References
-
- Apparsundaram S, Galli A, DeFelice LJ, Hartzell HC, Blakely RD. Acute regulation of norepinephrine transport: I. Protein kinase C-linked muscarinic receptors influence transport capacity and transporter density in SK-N-SH cells. J Pharmacol Exp Ther. 1998a;287:733–743. - PubMed
-
- Apparsundaram S, Schroeter S, Giovanetti E, Blakely RD. Acute regulation of norepinephrine transport: II. PKC-modulated surface expression of human norepinephrine transporter proteins. J Pharmacol Exp Ther. 1998b;287:744–751. - PubMed
-
- Barker EL, Blakely RD. Norepinephrine and serotonin transporters: molecular targets of antidepressant drugs. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven; New York: 1995. pp. 321–333.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
