Nckbeta adapter regulates actin polymerization in NIH 3T3 fibroblasts in response to platelet-derived growth factor bb
- PMID: 11027258
- PMCID: PMC86398
- DOI: 10.1128/MCB.20.21.7867-7880.2000
Nckbeta adapter regulates actin polymerization in NIH 3T3 fibroblasts in response to platelet-derived growth factor bb
Abstract
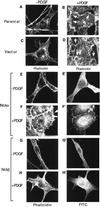
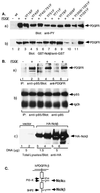
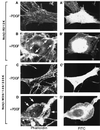
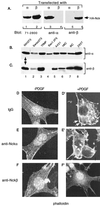
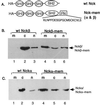
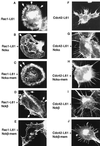
The SH3-SH3-SH3-SH2 adapter Nck represents a two-gene family that includes Nckalpha (Nck) and Nckbeta (Grb4/Nck2), and it links receptor tyrosine kinases to intracellular signaling networks. The function of these mammalian Nck genes has not been established. We report here a specific role for Nckbeta in platelet-derived growth factor (PDGF)-induced actin polymerization in NIH 3T3 cells. Overexpression of Nckbeta but not Nckalpha blocks PDGF-stimulated membrane ruffling and formation of lamellipoda. Mutation in either the SH2 or the middle SH3 domain of Nckbeta abolishes its interfering effect. Nckbeta binds at Tyr-1009 in human PDGF receptor beta (PDGFR-beta) which is different from Nckalpha's binding site, Tyr-751, and does not compete with phosphatidylinositol-3 kinase for binding to PDGFR. Microinjection of an anti-Nckbeta but not an anti-Nckalpha antibody inhibits PDGF-stimulated actin polymerization. Constitutively membrane-bound Nckbeta but not Nckalpha blocks Rac1-L62-induced membrane ruffling and formation of lamellipodia, suggesting that Nckbeta acts in parallel to or downstream of Rac1. This is the first report of Nckbeta's role in receptor tyrosine kinase signaling to the actin cytoskeleton.
Figures




References
-
- Birge R B, Knudsen B S, Besser D, Hanafusa H. SH2 and SH3-containing adaptor proteins: redundant or independent mediators of intracellular signal transduction. Genes Cells. 1996;1:595–613. - PubMed
-
- Braverman L E, Quilliam L A. Identification of Grb4/Nck, a src homology 2 and 3 domain-containing adapter protein having similar binding and biological properties to Nck. J Biol Chem. 1999;274:5542–5559. - PubMed
-
- Bubeck Wardenburg J, Pappu R, Bu J Y, Mayer B, Chernoff J, Straus D, Chan A C. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity. 1998;9:607–616. - PubMed
-
- Chen M, She H, Davis E M, Spicer C M, Kim L, Ren R, Le Beau M, Li W. Nck family genes, chromosomal location, expression and signaling specificity. J Biol Chem. 1998;273:25171–25178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
