The Ebola virus VP35 protein functions as a type I IFN antagonist
- PMID: 11027311
- PMCID: PMC17334
- DOI: 10.1073/pnas.220398297
The Ebola virus VP35 protein functions as a type I IFN antagonist
Abstract
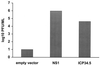
An assay has been developed that allows the identification of molecules that function as type I IFN antagonists. Using this assay, we have identified an Ebola virus-encoded inhibitor of the type I IFN response, the Ebola virus VP35 protein. The assay relies on the properties of an influenza virus mutant, influenza delNS1 virus, which lacks the NS1 ORF and, therefore, does not produce the NS1 protein. When cells are infected with influenza delNS1 virus, large amounts of type I IFN are produced. As a consequence, influenza delNS1 virus replicates poorly. However, high-efficiency transient transfection of a plasmid encoding a protein that interferes with type I IFN-induced antiviral functions, such as the influenza A virus NS1 protein or the herpes simplex virus protein ICP34.5, rescues growth of influenza delNS1 virus. When plasmids expressing individual Ebola virus proteins were transfected into Madin Darby canine kidney cells, the Ebola virus VP35 protein enhanced influenza delNS1 virus growth more than 100-fold. VP35 subsequently was shown to block double-stranded RNA- and virus-mediated induction of an IFN-stimulated response element reporter gene and to block double-stranded RNA- and virus-mediated induction of the IFN-beta promoter. The Ebola virus VP35 therefore is likely to inhibit induction of type I IFN in Ebola virus-infected cells and may be an important determinant of Ebola virus virulence in vivo.
Figures



References
-
- Klenk H-D, Slenczka W, Feldmann H. In: Encyclopedia of Virology. Webster R G, Granoff A, editors. Vol. 2. New York: Academic; 1994. pp. 827–831.
-
- Peters C J, Khan A S. Curr Top Microbiol Immunol. 1999;235:85–95. - PubMed
-
- Villinger F, Rollin P E, Brar S S, Chikkala N F, Winter J, Sundstrom J B, Zaki S R, Swanepoel R, Ansari A A, Peters C J. J Infect Dis. 1999;179, Suppl. 1:S188–S191. - PubMed
-
- Yang Z, Duckers H J, Sullivan N J, Sanchez A, Nabel E G, Nabel G J. Nat Med. 2000;6:886–889. - PubMed
-
- Yang Z, Delgado R, Xu L, Todd R F, Nabel E G, Sanchez A, Nabel G J. Science. 1998;279:1034–1037. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

