Cytoplasmic catalytic subunit of protein kinase A mediates cross-repression by NF-kappa B and the glucocorticoid receptor
- PMID: 11027313
- PMCID: PMC17265
- DOI: 10.1073/pnas.220413297
Cytoplasmic catalytic subunit of protein kinase A mediates cross-repression by NF-kappa B and the glucocorticoid receptor
Abstract
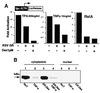
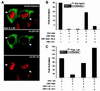
Negative transcriptional regulation or cross-coupling between NF-kappa B (RelA) and the glucocorticoid receptor (GR) is proposed to play a regulatory role in human physiology and disease. Despite previous advances, the biochemical basis of this phenomenon remains a subject of controversy. We show here that the inhibition of GR activity by RelA does not require the RelA DNA binding, transactivation, or nuclear localization domains. Surprisingly, RelA repression of GR is abolished by mutation of the conserved protein kinase A (PKA) site at amino acid residue 276 of RelA. We show that GR associates in vivo and in vitro with the catalytic subunit of PKA (PKAc) in a ligand-independent manner and that GR transcription depends on PKA signaling. Indeed, we demonstrated that GR-mediated inhibition of NF-kappa B transactivation is PKAc-dependent. In contrast to previous models, we suggest that the cross-coupling requires a cytoplasmic step and is regulated by a PKAc-associated signaling.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

