Nitric oxide prevents cardiovascular disease and determines survival in polyglobulic mice overexpressing erythropoietin
- PMID: 11027359
- PMCID: PMC17248
- DOI: 10.1073/pnas.97.21.11609
Nitric oxide prevents cardiovascular disease and determines survival in polyglobulic mice overexpressing erythropoietin
Abstract
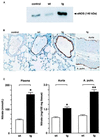
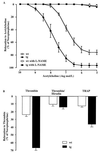
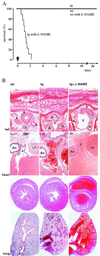
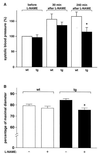
Nitric oxide (NO) induces vasodilatatory, antiaggregatory, and antiproliferative effects in vitro. To delineate potential beneficial effects of NO in preventing vascular disease in vivo, we generated transgenic mice overexpressing human erythropoietin. These animals induce polyglobulia known to be associated with a high incidence of vascular disease. Despite hematocrit levels of 80%, adult transgenic mice did not develop hypertension or thromboembolism. Endothelial NO synthase levels, NO-mediated endothelium-dependent relaxation and circulating and vascular tissue NO levels were markedly increased. Administration of the NO synthase inhibitor N(G)-nitro-L-arginine methyl ester (L-NAME) led to vasoconstriction of peripheral resistance vessels, hypertension, and death of transgenic mice, whereas wild-type siblings developed hypertension but did not show increased mortality. L-NAME-treated polyglobulic mice revealed acute left ventricular dilatation and vascular engorgement associated with pulmonary congestion and hemorrhage. In conclusion, we here unequivocally demonstrate that endothelial NO maintains normotension, prevents cardiovascular dysfunction, and critically determines survival in vivo under conditions of increased hematocrit.
Figures
References
-
- Vallance P, Collier J, Moncada S. Lancet. 1989;2:997–1000. - PubMed
-
- Pohl U, Holtz J, Busse R, Bassenge E. Hypertension. 1986;8:37–44. - PubMed
-
- Lüscher T F, Vanhoutte P M. The Endothelium: Modulator of Cardiovascular Function. Boca Raton, FL: CRC Press; 1990.
-
- Furchgott R F, Zawadzki J V. Nature (London) 1980;299:373–376. - PubMed
-
- Rapoport R M, Draznin M B, Murad F. Nature (London) 1983;306:174–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

