The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis
- PMID: 11027675
- PMCID: PMC2094390
- DOI: 10.1074/jbc.C000587200
The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis
Abstract
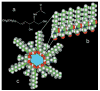
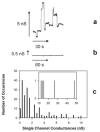
We report that physiological concentrations of both short- and long-chain ceramides, despite being lipids, form large stable pores in membranes. Some of these pores should be large enough to allow cytochrome c to permeate. Dihydroceramide differs from ceramide by the reduction of one double bond, and yet both its apoptogenic and channel-forming activities are greatly reduced. A structural model provides insight into how ceramides might form pores. According to a mathematical model, both the individual conductance of the channels and the overall membrane conductance are directly related to the overall concentration of ceramide in the membrane. Slight changes in concentration have dramatic effects on the size of the channels formed, providing an easy way for rapidly altering membrane permeability by changing the activity of local synthetic and catabolic enzymes. A possible role for these channels in apoptosis is discussed.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

