Abscisic acid stimulation of phospholipase D in the barley aleurone is G-protein-mediated and localized to the plasma membrane
- PMID: 11027718
- PMCID: PMC59174
- DOI: 10.1104/pp.124.2.693
Abscisic acid stimulation of phospholipase D in the barley aleurone is G-protein-mediated and localized to the plasma membrane
Abstract
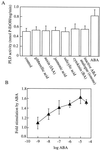
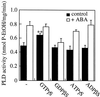
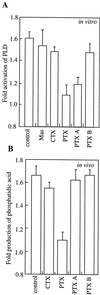
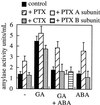
We have previously determined that phospholipase D (PLD) is activated by abscisic acid (ABA), and this activation is required for the ABA response of the cereal aleurone cell. In this study, ABA-stimulated PLD activity was reconstituted in vitro in microsomal membranes prepared from aleurone protoplasts. The transient nature (20 min) and degree (1.5- to 2-fold) of activation in vitro were similar to that measured in vivo. Stimulation by ABA was only apparent in the membrane fraction and was associated with a fraction enriched in plasma membrane. These results suggest that an ABA receptor system and elements linking it to PLD activation are associated with the aleurone plasma membrane. The activation of PLD in vitro by ABA was dependent on the presence of GTP. Addition of GTPgammaS transiently stimulated PLD in an ABA-independent manner, whereas treatment with GDPbetaS or pertussis toxin blocked the PLD activation by ABA. Application of pertussis toxin to intact aleurone protoplasts inhibited the ability of ABA to activate PLD as well as antagonizing the ability of ABA to down-regulate gibberellic acid-stimulated alpha-amylase production. All of these data support the hypothesis that ABA stimulation of PLD activity occurs at the plasma membrane and is mediated by G-protein activity.
Figures




Similar articles
-
Abscisic acid signal transduction in the barley aleurone is mediated by phospholipase D activity.Proc Natl Acad Sci U S A. 1998 Mar 3;95(5):2697-702. doi: 10.1073/pnas.95.5.2697. Proc Natl Acad Sci U S A. 1998. PMID: 9482950 Free PMC article.
-
Biotin-labeled abscisic acid as a probe for investigating abscisic acid binding sites on plasma membranes of barley aleurone protoplasts.Bioorg Med Chem. 2005 May 16;13(10):3351-8. doi: 10.1016/j.bmc.2005.03.017. Bioorg Med Chem. 2005. PMID: 15848747
-
Differences in phosphatidic acid signalling and metabolism between ABA and GA treatments of barley aleurone cells.Plant Physiol Biochem. 2013 Apr;65:1-8. doi: 10.1016/j.plaphy.2013.01.005. Epub 2013 Feb 4. Plant Physiol Biochem. 2013. PMID: 23416490
-
Abscisic acid induces a cytosolic calcium decrease in barley aleurone protoplasts.FEBS Lett. 1991 Jan 14;278(1):69-74. doi: 10.1016/0014-5793(91)80086-i. FEBS Lett. 1991. PMID: 1825201
-
Gibberellic acid and abscisic acid coordinately regulate cytoplasmic calcium and secretory activity in barley aleurone protoplasts.Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3591-5. doi: 10.1073/pnas.89.8.3591. Proc Natl Acad Sci U S A. 1992. PMID: 1533046 Free PMC article.
Cited by
-
Phosphoglycerolipids are master players in plant hormone signal transduction.Plant Cell Rep. 2013 Jun;32(6):839-51. doi: 10.1007/s00299-013-1399-0. Epub 2013 Mar 8. Plant Cell Rep. 2013. PMID: 23471417 Review.
-
Osmotically induced cell swelling versus cell shrinking elicits specific changes in phospholipid signals in tobacco pollen tubes.Plant Physiol. 2004 Feb;134(2):813-23. doi: 10.1104/pp.103.029454. Epub 2004 Jan 22. Plant Physiol. 2004. PMID: 14739344 Free PMC article.
-
Activation of phospholipases C and D is an early response to a cold exposure in Arabidopsis suspension cells.Plant Physiol. 2002 Oct;130(2):999-1007. doi: 10.1104/pp.006080. Plant Physiol. 2002. PMID: 12376663 Free PMC article.
-
Heterotrimeric Gα subunit from wheat (Triticum aestivum), GA3, interacts with the calcium-binding protein, Clo3, and the phosphoinositide-specific phospholipase C, PI-PLC1.Plant Mol Biol. 2011 Sep;77(1-2):145-58. doi: 10.1007/s11103-011-9801-1. Epub 2011 Jul 3. Plant Mol Biol. 2011. PMID: 21725861
-
Interaction of jasmonic acid with abscisic acid and gibberelic acid in the regulation of ATP-dependent proton translocation in plasmalemma vesicles from potato tuber cells.Dokl Biol Sci. 2003 Mar-Apr;389:163-5. doi: 10.1023/a:1023491430081. Dokl Biol Sci. 2003. PMID: 12854420 No abstract available.
References
-
- Assmann S. Guard cell G protein. Trends Plant Sci. 1996;1:73–74.
-
- Bethke PC, Schuurink R, Jones RL. Hormonal signaling in cereal aleurone. J Exp Bot. 1997;48:1337–1356.
-
- Bradford MM. A rapid and sensitive method for the quantitation of micrograms of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Brian PW. The effects of some microbial metabolic products on plant growth. Symp Soc Exp Biol. 1957;11:166–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

