Antisense suppression of 2-cysteine peroxiredoxin in Arabidopsis specifically enhances the activities and expression of enzymes associated with ascorbate metabolism but not glutathione metabolism
- PMID: 11027730
- PMCID: PMC59186
- DOI: 10.1104/pp.124.2.823
Antisense suppression of 2-cysteine peroxiredoxin in Arabidopsis specifically enhances the activities and expression of enzymes associated with ascorbate metabolism but not glutathione metabolism
Abstract
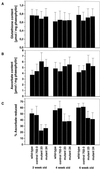
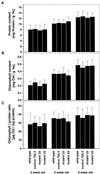
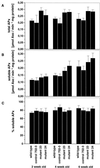
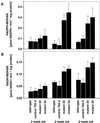
The aim of this study was to characterize the effect of decreased 2-cysteine peroxiredoxin (2-CP) on the leaf anti-oxidative system in Arabidopsis. At three stages of leaf development, two lines of transgenic Arabidopsis mutants with decreased contents of chloroplast 2-CP were compared with wild type and a control line transformed with an empty vector. Glutathione contents and redox state were similar in all plants, and no changes in transcript levels for enzymes involved in glutathione metabolism were observed. Transcript levels for chloroplastic glutathione peroxidase were much lower than those for 2-CP, and both cytosolic and chloroplastic glutathione peroxidase were not increased in the mutants. In contrast, the foliar ascorbate pool was more oxidized in the mutants, although the difference decreased with plant age. The activities of thylakoid and stromal ascorbate peroxidase and particularly monodehydroascorbate reductase were increased as were transcripts for these enzymes. No change in dehydroascorbate reductase activity was observed, and effects on transcript abundance for glutathione reductase, catalase, and superoxide dismutase were slight or absent. The results demonstrate that 2-CP forms an integral part of the anti-oxidant network of chloroplasts and is functionally interconnected with other defense systems. Suppression of 2-CP leads to increased expression of other anti-oxidative genes possibly mediated by increased oxidation state of the leaf ascorbate pool.
Figures


References
-
- Allen CA, Håkansson G, Allen JF. Redox conditions specify the protein synthesised by isolated chloroplasts and mitochondria. Redox Rep. 1995;1:119–123. - PubMed
-
- Allen JF, Nilsson A. Redox signalling and the structural basis of regulation of photosynthesis by protein phosphorylation. Physiol Plant. 1997;100:863–868.
-
- Asada K. The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. - PubMed
-
- Baier M, Dietz K-J. Primary structure and expression of plant homologues of animal and fungal thioredoxin-dependent peroxide reductases and bacterial alkyl hydroperoxide reductases. Plant Mol Biol. 1996;31:553–564. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

