Protection of telomeres by the Ku protein in fission yeast
- PMID: 11029034
- PMCID: PMC14990
- DOI: 10.1091/mbc.11.10.3265
Protection of telomeres by the Ku protein in fission yeast
Abstract
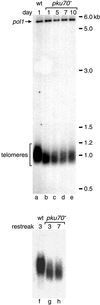
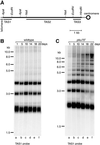
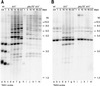
Schizosaccharomyces pombe cells survive loss of telomeres by a unique pathway of chromosome circularization. Factors potentially involved in this survival mechanism include the heterodimeric Ku protein and ligase IV, both of which are involved in the repair of DNA double-strand breaks in mammalian cells. Furthermore, Ku plays a role in telomere maintenance as well as in DNA double-strand break repair in Saccharomyces cerevisiae. We have identified Ku and ligase IV homologues in S. pombe and analyzed their functions during normal growth and in cells undergoing senescence. In the absence of either a Ku subunit (pku70(+)) or ligase IV (lig4(+)), nonhomologous DNA end-joining was severely reduced. Lack of functional Ku led to shorter but stable telomeres and caused striking rearrangements of telomere-associated sequences, indicating a function for Ku in inhibiting recombinational activities near chromosome ends. In contrast to S. cerevisiae, concurrent deletion of pku70(+) and the gene for the catalytic subunit of telomerase (trt1(+)) was not lethal, allowing for the first time the dissection of the roles of Ku during senescence. Our results support a model in which Ku protects chromosome termini from nucleolytic and recombinational activities but is not involved in the formation of chromosome end fusions during senescence. The conclusion that nonhomologous end-joining is not required for chromosome circularization was further supported by analysis of survivors in strains lacking the genes for both trt1(+) and lig4(+).
Figures




References
-
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with Fission Yeast. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1993.
-
- Bianchi A, de Lange T. Ku binds telomeric DNA in vitro. J Biol Chem. 1999;274:21223–21227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

