The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes
- PMID: 11029036
- PMCID: PMC14992
- DOI: 10.1091/mbc.11.10.3289
The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes
Abstract
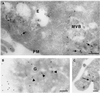
Endobrevin/VAMP-8 is an R-SNARE localized to endosomes, but it is unknown in which intracellular fusion step it operates. Using subcellular fractionation and quantitative immunogold electron microscopy, we found that endobrevin/VAMP-8 is present on all membranes known to communicate with early endosomes, including the plasma membrane, clathrin-coated pits, late endosomes, and membranes of the trans-Golgi network. Affinity-purified antibodies that block the ability of endobrevin/VAMP-8 to form SNARE core complexes potently inhibit homotypic fusion of both early and late endosomes in vitro. Fab fragments were as active as intact immunoglobulin Gs. Recombinant endobrevin/VAMP-8 inhibited both fusion reactions with similar potency. We conclude that endobrevin/VAMP-8 operates as an R-SNARE in the homotypic fusion of early and late endosomes.
Figures





References
-
- Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. - PubMed
-
- Barnstable CJ, Hofstein R, Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352:286–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

