Forced expression of keratin 16 alters the adhesion, differentiation, and migration of mouse skin keratinocytes
- PMID: 11029038
- PMCID: PMC14994
- DOI: 10.1091/mbc.11.10.3315
Forced expression of keratin 16 alters the adhesion, differentiation, and migration of mouse skin keratinocytes
Abstract
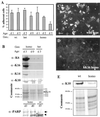
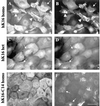
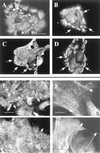
Injury to the skin results in an induction of keratins K6, K16, and K17 concomitant with activation of keratinocytes for reepithelialization. Forced expression of human K16 in skin epithelia of transgenic mice causes a phenotype that mimics several aspects of keratinocyte activation. Two types of transgenic keratinocytes, with forced expression of either human K16 or a K16-C14 chimeric cDNA, were analyzed in primary culture to assess the impact of K16 expression at a cellular level. High K16-C14-expressing and low K16-expressing transgenic keratinocytes behave similar to wild type in all aspects tested. In contrast, high K16-expressing transgenic keratinocytes show alterations in plating efficiency and calcium-induced differentiation, but proliferate normally. Migration of keratinocytes is reduced in K16 transgenic skin explants compared with controls. Finally, a subset of high K16-expressing transgenic keratinocytes develops major changes in the organization of keratin filaments in a time- and calcium concentration-dependent manner. These changes coincide with alterations in keratin content while the steady-state levels of K16 protein remain stable. We conclude that forced expression of K16 in progenitor skin keratinocytes directly impacts properties such as adhesion, differentiation, and migration, and that these effects depend upon determinants contained within its carboxy terminus.
Figures




Similar articles
-
Increased levels of keratin 16 alter epithelialization potential of mouse skin keratinocytes in vivo and ex vivo.Mol Biol Cell. 2001 Nov;12(11):3439-50. doi: 10.1091/mbc.12.11.3439. Mol Biol Cell. 2001. PMID: 11694579 Free PMC article.
-
Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16.J Cell Biol. 1996 Feb;132(3):381-97. doi: 10.1083/jcb.132.3.381. J Cell Biol. 1996. PMID: 8636216 Free PMC article.
-
The functional diversity of epidermal keratins revealed by the partial rescue of the keratin 14 null phenotype by keratin 16.J Cell Biol. 1999 Sep 6;146(5):1185-201. doi: 10.1083/jcb.146.5.1185. J Cell Biol. 1999. PMID: 10477769 Free PMC article.
-
Overexpression of human keratin 16 produces a distinct skin phenotype in transgenic mouse skin.Biochem Cell Biol. 1995 Sep-Oct;73(9-10):611-8. doi: 10.1139/o95-067. Biochem Cell Biol. 1995. PMID: 8714680 Review.
-
Discovery of keratin function and role in genetic diseases: the year that 1991 was.Mol Biol Cell. 2016 Sep 15;27(18):2807-10. doi: 10.1091/mbc.E15-09-0625. Mol Biol Cell. 2016. PMID: 27634744 Free PMC article. Review.
Cited by
-
Increased levels of keratin 16 alter epithelialization potential of mouse skin keratinocytes in vivo and ex vivo.Mol Biol Cell. 2001 Nov;12(11):3439-50. doi: 10.1091/mbc.12.11.3439. Mol Biol Cell. 2001. PMID: 11694579 Free PMC article.
-
Differential keratin expression during epiboly in a wound model of bioengineered skin and in human chronic wounds.Int J Low Extrem Wounds. 2011 Sep;10(3):122-9. doi: 10.1177/1534734611418157. Epub 2011 Aug 19. Int J Low Extrem Wounds. 2011. PMID: 21856973 Free PMC article.
-
Emerging Insights into Keratin 16 Expression during Metastatic Progression of Breast Cancer.Cancers (Basel). 2021 Jul 31;13(15):3869. doi: 10.3390/cancers13153869. Cancers (Basel). 2021. PMID: 34359774 Free PMC article.
-
The nonhelical tail domain of keratin 14 promotes filament bundling and enhances the mechanical properties of keratin intermediate filaments in vitro.J Cell Biol. 2001 Nov 26;155(5):747-54. doi: 10.1083/jcb.200104063. Epub 2001 Nov 26. J Cell Biol. 2001. PMID: 11724817 Free PMC article.
-
Vimentin is necessary for colony growth of human diploid keratinocytes.Histochem Cell Biol. 2015 Jan;143(1):45-57. doi: 10.1007/s00418-014-1262-6. Epub 2014 Aug 21. Histochem Cell Biol. 2015. PMID: 25142512
References
-
- Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin α3-β1 in epithelial basements membranes. Cell. 1991;65:599–610. - PubMed
-
- Clark RAF. Mechanisms of cutaneous wound repair. In: Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen, M.D MD, editors. Dermatology in General Medicine. I. New York: McGraw-Hill Book Company; 1993. pp. 473–486.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

