Rotational coupling of the transmembrane and kinase domains of the Neu receptor tyrosine kinase
- PMID: 11029057
- PMCID: PMC15017
- DOI: 10.1091/mbc.11.10.3589
Rotational coupling of the transmembrane and kinase domains of the Neu receptor tyrosine kinase
Abstract
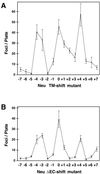
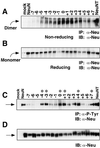
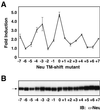
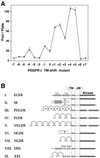
Ligand binding to receptor tyrosine kinases (RTKs) regulates receptor dimerization and activation of the kinase domain. To examine the role of the transmembrane domain in regulation of RTK activation, we have exploited a simplified transmembrane motif, [VVVEVVV](n), previously shown to activate the Neu receptor. Here we demonstrate rotational linkage of the transmembrane domain with the kinase domain, as evidenced by a periodic activation of Neu as the dimerization motif is shifted across the transmembrane domain. These results indicate that activation requires a specific orientation of the kinase domains with respect to each other. Results obtained with platelet-derived growth factor receptor-beta suggest that this rotational linkage of the transmembrane domain to the kinase domain may be a general feature of RTKs. These observations suggest that activating mutations in RTK transmembrane and juxtamembrane domains will be limited to those residues that position the kinase domains in an allowed rotational conformation.
Figures



References
-
- Bargmann CI, Hung M-C, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986a;319:226–230. - PubMed
-
- Bargmann CI, Hung M-C, Weinberg RA. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986b;45:649–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

