Yeast exocytic v-SNAREs confer endocytosis
- PMID: 11029060
- PMCID: PMC15020
- DOI: 10.1091/mbc.11.10.3629
Yeast exocytic v-SNAREs confer endocytosis
Abstract
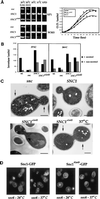
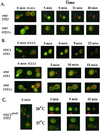
In yeast, homologues of the synaptobrevin/VAMP family of v-SNAREs (Snc1 and Snc2) confer the docking and fusion of secretory vesicles at the cell surface. As no v-SNARE has been shown to confer endocytosis, we examined whether yeast lacking the SNC genes, or possessing a temperature-sensitive allele of SNC1 (SNC1(ala43)), are deficient in the endocytic uptake of components from the cell surface. We found that both SNC and temperature-shifted SNC1(ala43) yeast are deficient in their ability to deliver the soluble dye FM4-64 to the vacuole. Under conditions in which vesicles accumulate, FM4-64 stained primarily the cytoplasm as well as fragmented vacuoles. In addition, alpha-factor-stimulated endocytosis of the alpha-factor receptor, Ste2, was fully blocked, as evidenced using a Ste2-green fluorescent protein fusion protein as well as metabolic labeling studies. This suggests a direct role for Snc v-SNAREs in the retrieval of membrane proteins from the cell surface. Moreover, this idea is supported by genetic and physical data that demonstrate functional interactions with t-SNAREs that confer endosomal transport (e.g., Tlg1,2). Notably, Snc1(ala43) was found to be nonfunctional in cells lacking Tlg1 or Tlg2. Thus, we propose that synaptobrevin/VAMP family members are engaged in anterograde and retrograde protein sorting steps between the Golgi and the plasma membrane.
Figures

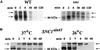


Similar articles
-
t-SNARE phosphorylation regulates endocytosis in yeast.Mol Biol Cell. 2002 May;13(5):1594-607. doi: 10.1091/mbc.01-11-0541. Mol Biol Cell. 2002. PMID: 12006655 Free PMC article.
-
Conserved alpha-helical segments on yeast homologs of the synaptobrevin/VAMP family of v-SNAREs mediate exocytic function.J Biol Chem. 1997 Jun 27;272(26):16591-8. doi: 10.1074/jbc.272.26.16591. J Biol Chem. 1997. PMID: 9195971
-
A snc1 endocytosis mutant: phenotypic analysis and suppression by overproduction of dihydrosphingosine phosphate lyase.Mol Biol Cell. 2000 Dec;11(12):4051-65. doi: 10.1091/mbc.11.12.4051. Mol Biol Cell. 2000. PMID: 11102507 Free PMC article.
-
SNARE Protein Snc1 Is Essential for Vesicle Trafficking, Membrane Fusion and Protein Secretion in Fungi.Cells. 2023 Jun 5;12(11):1547. doi: 10.3390/cells12111547. Cells. 2023. PMID: 37296667 Free PMC article. Review.
-
Genetic analysis of clathrin function in yeast.J Membr Biol. 1990 Jun;116(2):93-105. doi: 10.1007/BF01868668. J Membr Biol. 1990. PMID: 2199679 Review.
Cited by
-
A t-SNARE of the endocytic pathway must be activated for fusion.J Cell Biol. 2001 Dec 10;155(6):961-8. doi: 10.1083/jcb.200104092. Epub 2001 Dec 10. J Cell Biol. 2001. PMID: 11739407 Free PMC article.
-
The Gcs1 Arf-GAP mediates Snc1,2 v-SNARE retrieval to the Golgi in yeast.Mol Biol Cell. 2006 Apr;17(4):1845-58. doi: 10.1091/mbc.e05-09-0832. Epub 2006 Feb 1. Mol Biol Cell. 2006. PMID: 16452633 Free PMC article.
-
t-SNARE dephosphorylation promotes SNARE assembly and exocytosis in yeast.EMBO J. 2001 Feb 1;20(3):411-21. doi: 10.1093/emboj/20.3.411. EMBO J. 2001. PMID: 11157748 Free PMC article.
-
Actin and endocytosis in budding yeast.Genetics. 2015 Feb;199(2):315-58. doi: 10.1534/genetics.112.145540. Genetics. 2015. PMID: 25657349 Free PMC article. Review.
-
Exocyst stimulates multiple steps of exocytic SNARE complex assembly and vesicle fusion.Nat Struct Mol Biol. 2025 Jan;32(1):150-160. doi: 10.1038/s41594-024-01388-2. Epub 2024 Sep 6. Nat Struct Mol Biol. 2025. PMID: 39242980
References
-
- Abeliovich H, Grote E, Novick P, Ferro-Novick S. Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J Biol Chem. 1998;273:11719–11727. - PubMed
-
- Brennwald P, Kearns B, Champion K, Keranen S, Bankaitis V, Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

