Wpkci, encoding an altered form of PKCI, is conserved widely on the avian W chromosome and expressed in early female embryos: implication of its role in female sex determination
- PMID: 11029061
- PMCID: PMC15021
- DOI: 10.1091/mbc.11.10.3645
Wpkci, encoding an altered form of PKCI, is conserved widely on the avian W chromosome and expressed in early female embryos: implication of its role in female sex determination
Abstract
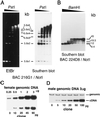
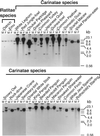
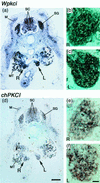
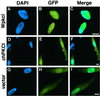
Two W chromosome-linked cDNA clones, p5fm2 and p5fm3, were obtained from a subtracted (female minus male) cDNA library prepared from a mixture of undifferentiated gonads and mesonephroi of male or female 5-d (stages 26-28) chicken embryos. These two clones were demonstrated to be derived from the mRNA encoding an altered form of PKC inhibitor/interacting protein (PKCI), and its gene was named Wpkci. The Wpkci gene reiterated approximately 40 times tandemly and located at the nonheterochromatic end of the chicken W chromosome. The W linkage and the moderate reiteration of Wpkci were conserved widely in Carinatae birds. The chicken PKCI gene, chPKCI, was shown to be a single-copy gene located near the centromere on the long arm of the Z chromosome. Deduced amino acid sequences of Wpkci and chPKCI showed approximately 65% identity. In the deduced sequence of Wpkci, the HIT motif, which is essential for PKCI function, was absent, but the alpha-helix region, which was conserved among the PKCI family, and a unique Leu- and Arg-rich region, were present. Transcripts from both Wpkci and chPKCI genes were present at significantly higher levels in 3- to 6-d (stages 20-29) embryos. These transcripts were detected in several embryonic tissues, including undifferentiated left and right gonads. When the green fluorescent protein-fused form of Wpkci was expressed in male chicken embryonic fibroblast, it was located almost exclusively in the nucleus. A model is presented suggesting that Wpkci may be involved in triggering the differentiation of ovary by interfering with PKCI function or by exhibiting its unique function in the nuclei of early female embryos.
Figures








References
-
- Asakawa S, Abe I, Kudoh Y, Kishi N, Wang Y, Kubota R, Kudoh J, Kawasaki K, Minoshima S, Shimizu N. Human BAC library: construction and rapid screening. Gene. 1997;20:69–79. - PubMed
-
- Barber DL, Sanders EJ, Aebersold R, Schneider WJ. The receptor for yolk lipoprotein deposition in the chicken oocyte. J Biol Chem. 1991;266:18761–18770. - PubMed
-
- Brzoska PM, Chen H, Levin NA, Kuo WL, Collins C, Fu KK, Gray JW, Christman MF. Cloning, mapping, and in vivo localization of a human member of the PKCI-1 protein family (PRKCNH1) Genomics. 1996;36:151–156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

